Biomanufacturing is moving towards higher efficiency through process intensification. One aspect of intensification is achieving operational excellence by connecting unit operations and running the process functionally closed. The advantages of closed processing are shorter processing time, less manual interactions in the process, reduced CAPEX, reduced resources for cleaning and validation, and reduced need for qualified manufacturing space.
Closed connected processing, however, poses new challenges with respect to process development, planning, and execution. This white paper reviews the benefits and challenges with closed processing and connected processing. By giving examples for the implementation of closed, connected processing in large-scale manufacturing, you will be able to explore and assess options that work for your objectives.
Design considerations
Biomanufacturing is under increased pressure to adapt to the market demands by reducing costs, increasing efficiencies, increasing speed to clinic trials and market, and to design flexible, multi-product facilities. During the last few years, process intensification has been the focus to increase efficiencies while ensuring product quality. In 2019, the FDA issued a draft guidance on continuous manufacturing. This was focused on small molecules, but there was content covering biologics. Ahead of this draft guidance, several continuous processes have been approved for marketed small molecule products.
As individual unit operations are debottlenecked, focus has shifted to consider efficiency across an entire manufacturing process. These approaches, especially connected and continuous or semi-continuous processes, can reduce equipment footprint, CAPEX, and production costs, while maintaining product quality and manufacturing throughput. In addition, a closed process, where the product is protected from the environment by the process being aseptic throughout, has the potential to improve process efficiency further.
However, planning and performing a closed connected process requires significant effort be placed into its design considerations. For a well-designed process knowing the mass balance for each unit operation as well as the critical quality attributes is key. This knowledge will dictate batch sizes, equipment sizes, and suitable technologies and materials for final scale. Aligning the design space of multiple processes with a well-characterized operating space across the start to finish manufacturing process will ensure the operational flexibility required and a successful high-quality product. This white paper will provide information on how to avoid the major pitfalls when designing and executing a semi-continuous or continuous, closed connected bioprocess.
Definitions
These definitions reference external sources including a white paper from the Continuous Manufacturing Symposium (2014), BPOG’s guidance document (2019), and ISPE’s Biopharmaceutical Manufacturing Facilities Baseline® Guide (2013).
A closed system is designed and operated such that the product is not exposed to the operations environment. Materials can be introduced into the system; however, it must be done in such a way as to avoid exposing the product. System closure is often defined by the boundary in which the product can be separated from the environment and may be different than the physical boundaries of process equipment. Systems may be intrinsically closed in that they are capable of generating or creating a sterile boundary, for instance sterile single-use connectors which do not expose the internal flow path to the environment during assembly. Systems may also be “rendered functionally closed” through manipulation of flow paths, for instance steaming a flow path and maintaining pressure on the product contact surfaces until used.
In a connected process, two or more-unit operations are physically connected. The unit operations may be batch, continuous, or a mix. Planned product hold steps, hold-up volumes in between, and manual interventions are minimized or eliminated. Discrete product quality states may exist in between unit operations.
A semi-continuous process is a process where continuous and batch unit operations are combined. This may also be referred to as a hybrid process, however the authors recommend this term for the more commonly adopted definition applied to processes consisting of a mix of stainless steel and single-use unit operations.
In a continuous process, multiple continuous unit operations are connected into an integrated set-up. Examples of continuous unit operations include production cell culture using perfusion, product capture using periodic counter-current chromatography (PCC), and single-pass tangential flow filtration (SPTFF). In a continuous process, the product of interest is constantly transforming and therefore product quality is defined by a time continuum rather than the sampling location.
What is a closed connected process?
A complete process to produce a biopharmaceutical consists of many unit operations. Linking these different steps together into a connected process can be a challenging task. One advantage of using a connected bioprocess is to increase facility utilization, a key driver of cost. For example, perfusion cell culture makes it possible to transfer the material directly to the capture step without any mid-stream centrifugation. A perfusion cell culture can also reduce the required floor space, as smaller bioreactors can be used. However, proper media and process liquid handling considerations becomes more important.
A closed system reduces the risk of contamination due to the physical and operational barriers put in place to protect the product from human and environmental contact. Due to this, closed systems reduce the operating time necessary as there is less handling by operators in the execution phase. A closed system is primarily achieved by using single-use sterile consumables, which reduces the time needed for cleaning, validation, and setup. These single-use consumables are delivered from the supplier pre-sterilized.
One design consideration for a continuous process is the use of a surge tanks between upstream and downstream operations. Ideally, the product movement within a continuous manufacturing line would occur at the same rate. Without surge tanks the entire process would be rate limited by the slowest unit operations in the process. Surge tanks allow unit operations to go idle without slowing down or terminating adjacent unit operations or the entire process. Therefore, it can also enable maintenance activities to occur, reducing premature process termination.
Example of a closed connected process
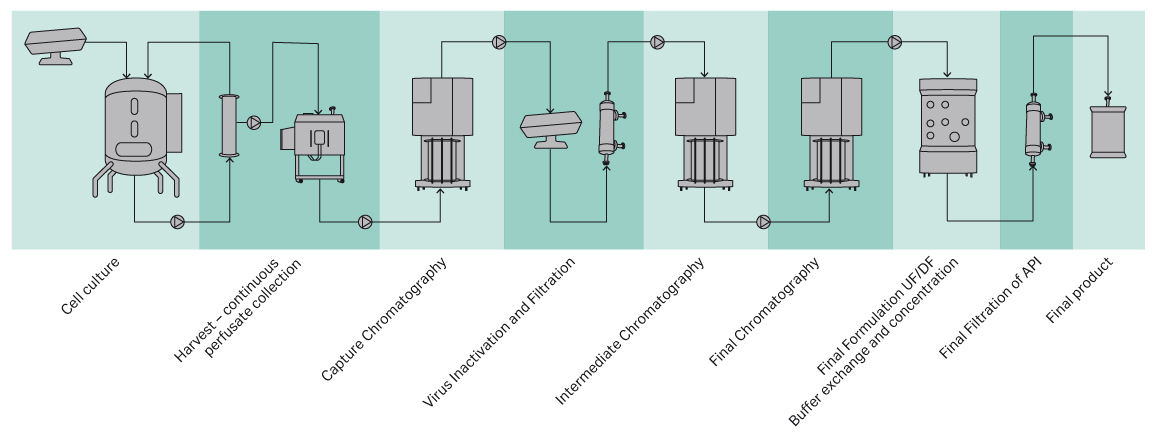
Continuous processing has been used in upstream processing for many decades through the use of perfusion processes to produce unstable proteins. This experience base can be leveraged for general implementation of next generation processes. Recent advances in downstream continuous processing include continuous chromatography, conditioning, and filtration unit operations. An example process flow diagram of a closed continuous process is shown in Figure 1.
Fig 1. Example schematic of a closed connected process.
Closed processing has always been mandatory in upstream processing to maintain the sterility and to avoid contaminations. Also, an ecosystem of closed vessels or bags for media, feeds, glucose, antifoam, etc., have been well established. Continuous upstream processes (i.e., perfusion) has a long history in the production of unstable proteins and is therefore well-established. Filter-based cell retention devices in perfusion processes offer the advantage of delivering a cell-free harvest to the subsequent capture step.
Often, a small tank or bag is located between the upstream process and the capture in order to compensate for differences or changes in the perfusion feed rates, provide surge capacity, and to store volume in case of down-time of the capture skid.
The capture step may be operated in a closed system or rendered functionally closed either in continuous mode, e.g., using periodic countercurrent chromatography (PCC) or in batch mode. Developing a cycled or periodic CIP of the capture column in a closed set-up is typically sufficient to maintain bacteriostatic operations. One system design consideration related to closed processing is the handling of waste streams. In classified spaces, waste discharge is often done atmospherically to the room environment. Defense of this type of discharge usually includes system designs which limit the exposure to specific areas of the manufacturing area and/or operational controls to ensure positive system pressure on the product contact flow path and consideration of other unit operation sequences to minimize risk of cross-contamination.
The polishing steps often are not rate limiting and therefore more conducive to elimination of operating manipulations, surge vessels between unit operations, and minimization of the hold-up volume, sometimes to the degree of combining multiple unit operations into a single system design. Examples of this include combination of bind and elute and flow through chromatography steps, flow through chromatography steps, and chromatography and filtration steps.
The retentate vessel for tangential flow filtration is essentially a pool collection vessel prior to starting the initial concentration step. However, by using the mass balance and understanding the coordination of product movement in prior unit operations and incorporating connected processing can reduce the vessel working volume and thereby reducing the overall system footprint.
Critical enablers for closed connected processing
The most important design consideration to analyze is the mass balance of the process. The mass balance of the system will dictate the sizing of the equipment, instruments, columns, buffer and media volumes, recovery, and product yield. A good point to start is at the end of the process to correctly scale it to support your product need. In traditional batch processes, the mass balance is considered as a discrete value for each unit operation, and mismatches between equipment is managed through intermediate pool vessels. In connected processes, intermediate pools are eliminated or reduced to surge capacity, and therefore alignment of the unit operations in terms of scale and throughput becomes imperative. Single unit operation knowledge can still be leveraged for connected process calculations. Fundamental parameters to consider are:
| Application |
Parameter |
| Product Amount | Titer Yield Totalized amount based on UV |
| Product Movement | Flow rate Residence time UV |
There are several different process technologies that are critical for implementing a closed connected process. The first is single-use technology (SUT) is a critical enabler for closed processing as a closed flow path can be designed with single-use components, i.e., bioreactors, tubing, manifolds, bags, filters, chromatography columns and chromatography system.
In a closed process, it is important to aseptically connect the unit operations using aseptic connectors. Aseptic connectors are available as both connect and disconnect variants and are single-use. In cases where suitable connectors cannot be employed, careful design and operational defense of the physical connections are required. In cases where aseptic connections are not available, the system may be “rendered closed” or deemed “functionally closed”, for instance by performing a pre-use cleaning across the surfaces of open connections prior to use.
Less samples are drawn and analyzed in a closed process. As a result, more sensors, preferably as single-use are required to monitor the process. Examples of available single-use sensors are capacitance sensors to monitor the viable cell density upstream, disposable flow meters, load cells, pressure sensors, pH and conductivity sensors. Using process trend surrogates or positioning laboratory analytical methods in a GMP manufacturing setting are ways in which the target product can be monitored and measured throughout the process.
Process automation together with sensors is an important enabler for closed, connected processing. When unit operations are connected, there is a need for both feed-back and feed-forward control. Using a nominal mass balance can help align adjacent unit operations in terms of matching flow rates, operational sequences, and as experience is built, monitoring and alarming strategies as well as restart and recovery from process deviations.
Modern facilities are often designed as ballroom controlled nonclassified (CNC) space. That means that both upstream and downstream unit operations are performed in the same, large suite without physical segregation and without cleanroom classification. The ability to avoid open handling and to contain the product in a closed flow path is the prerequisite for this design, but also for the capability to successfully pass inspection of the manufacturing space.
As previously mentioned, employing a connected process increases the facility utilization, and results in a proportional increase in the use rate of process feed materials such as cell culture media and purification buffers. Process solution management should be considered carefully as their scale of use is higher. There are several intensification options available such as:
- direct supply of use-strength solutions from third parties,
- kitted and concentrated formulas which only require hydration or dilution when used, and just-in-time deliver through advanced system designs, such as inline conditioning systems which use solution stocks rather than titrated concentrates.
Challenges for connected processing
Even though closed connected processing has already been implemented in the industry by early adopters, certain challenges remain. One major challenge is related to the automation and the implementation of feed-back and feed-forward control. These features are necessary to match the variance in volume and mass flow through the process over time. Some of the variance is induced by the biologic system (i.e., the cell culture process) in the upstream part, however, downstream process stream compositions potentially change over time with respect to yield and impurity. The cascade or priority given to multiple control loops also presents an iterative design activity that requires experience to finalize. Sensors to specifically measure the critical quality attributes are lacking which could be used for process control. Another challenge is related to connectors, as many connectors can only be connected once. This requires a high amount of forward planning to foresee all connections that might be needed during the process and the appropriate order in which to make them. Finally, there are limited technical solutions available for drug substance (DS) formulation by means of UF/DF that are single-use and enable closed and connected set-up at large scale.
Recommendations and design considerations
Objectives to set your strategy
Connected processes should not be pursued for the sake of a unique or novel technology, but rather the process design and strategy should meet overall objectives, goals, and timelines. The details needed to set your objectives include the manufacturing network you plan to deploy to, the product mix and campaign plan within your network, process platform scalability, technology transfer knowledge, and timeline. Some questions that arise during this consideration include what your internal strengths and challenges are, as well as those of external partners. While your objectives often focus on delivering product to patients, they should also include the age and functionality of your manufacturing facilities and incorporate opportunities to modernize.
Technology selection
The first set of decision points encountered often involve what process technologies to evaluate and implement into your manufacturing process. A flexible integration approach requires both automation expertise and bioprocess knowledge, however selection is often influenced by what is familiar, readily available, and/or relevant to the manufacturing objectives. Some options may carry more risk, and therefore require more development activities to understand and implement. Using batch experience can balance the seeming need for “extra” process development with timing and resources. Additionally, parallel activities ahead of decision points can allow for contingencies to be developed prior to process definition and lock.
Usually an initial technology decision that will have a lasting impact is related to the automation platform and approach. Various levels of automation are available with different capabilities and price points. Understanding the options for cross-unit communication, trending, and control is key for selection of the appropriate solution for the project objectives. For instance, will equipment communicate with each other, or will personnel manipulate the equipment based on aggregated process trends. The key risks and failure modes for not only each unit operation, but also adjacent unit operations will also be key in decision making.
Planning a semi-continuous or continuous process
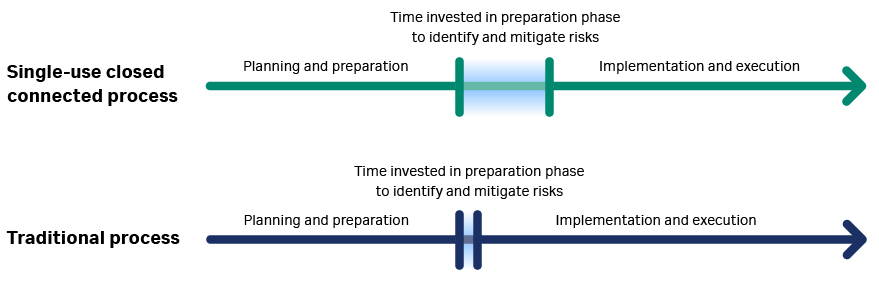
Prior to planning a connected process, the development steps necessary for scale-up should be performed. For example, the hold time studies for product, buffer/media compositions and volumes, product intermediate volumes, and process settings should be known. To ensure that everything is planned correctly it is helpful to do a process simulation both on paper and as line runs in the lab, as shown in Figure 2. This also helps to guide the bill of materials (BOM) list and orders for materials that will be needed.
As shown in Figure 2, the focus of designing, executing, and implementing a closed connected single-use process should be on the planning and preparation phases. This will help to anticipate and mitigate risks for a seamless final execution of the process.
Fig 2. Comparison of timelines for the implementation of a traditional bioprocess and closed connected process.
Physical space planning
Once your process technology is selected and scaled, it is necessary to consider the available laboratory or cleanroom space and equipment footprint. It is required to ensure that with the given equipment footprints there is enough space to move personnel and materials around, accessing key points on the equipment during the run. In addition, utility (gas, water, and electricity) connections, and material flows for liquid and solid waste need to be considered. There should be enough space to enable independent operation of upstream and downstream unit operations. In addition, this planning needs to be extended to warehouse capacity. There should be enough space in freezers and cold rooms for storage of product intermediates and samples.
Fig 3. Run material staging before unpacking.
Fig 4. Run material staging after unpacking.
Logistics planning
To ensure that consumables are on hand during runs, it is important that consumables are ordered on time, considering single-use consumable delivery times and to have a good inventory management system in place. It is also advisable that there is a safety margin on critical consumables, e.g., bioreactor bags. A plan for the consumables used per step or per day is recommended, as well as planning where consumables will be stored. Staging the consumables according to their use is also recommended to ensure timely transfer to the manufacturing operation.
The daily activities for the staff (e.g., sampling, media preparation, etc.) and the different unit operations should be planned. The right competencies should be staffed at the right place, and mandates should be developed for making decisions during deviations, alarms or process related issues. As a closed connected process is implemented, facility managers should ensure that operators are trained on the new process. It is important to include operators and engineers in the process design, as this new approach can be meet with uncertainty.
Risk analysis
An important part of the planning phase is to perform a risk analysis. A risk analysis should include relevant risks that are critical for the process, and mitigations to minimize risks during the planning and preparation phases. Risk mitigation should continue throughout the process, with critical risks being periodically reviewed. Some risks are inherent but having a risk analysis done will help to anticipate risks and have backup plans.
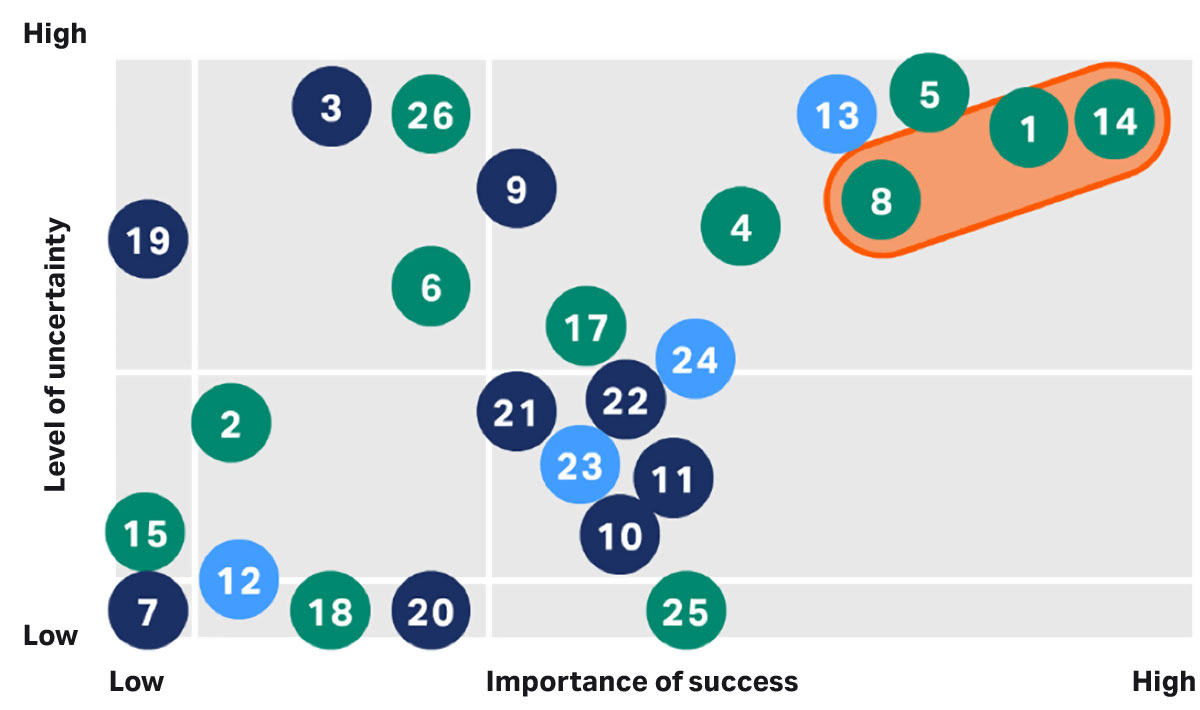
Fig 5. Leap of Faith Assumptions (LoFA) is a useful visualization tool for risk assessments.
Small-scale studies can greatly inform a formal risk assessment activity and lead to higher likelihood of a successful scale-up. Anticipating and designing risk mitigation can take place in a controlled proactive manner instead of as a result of large-scale deviation. Included in this assessment should be details for process closures to ensure appropriate design of the process flow as well as sharing information across the operations team. The overall risk should also translate into an early decision and plan for engineering studies at the final scale. The trend has been to minimize these large-scale activities since they are time-consuming and costly, but for connected processes, it is highly recommended to perform engineering runs.
Execution
An advantage of a closed system is that many of the components, such as buffers, media, and consumables, can be prepared and aseptically connected in advance of a run. This reduces the workload that is necessary during the run. However, it also requires that these materials are organized and stored properly in preparation, i.e., the preparation and equipment set-up is often intensified as much as the process. Reconnection is often not possible once connections are made. To overcome this and improve flexibility, redundancies should be placed in the systems, such as extra manifolds, Y connections, and bags.
Prior to starting the process, alarm limits for important process parameters should be defined and methods for measurement should be chosen. One way to make runs more efficient and to ensure that critical process parameters do not go outside of their acceptable ranges is to use in-line sensors. In-line sensors can be used to automate processes by using a feedback loop. Some examples of process that can be automated by using in-line sensors are given in Table 1.
Table 1. Opportunities for automation and in-line sensor implementation
| Sensor |
Principle of measurement |
Process application |
| Biomass | Capacitance | Cells specific perfusion rate control Bleed rate control Inoculum transfer |
| Load cell | Mass | Volume control surge vessels Columns loading from surge vessels Virus inactivation |
| pH | pH | Virus inactivation Conditioning prior to polishing load |
| Conductivity | Conductivity | Conditioning prior to polishing load |
Visualization and Analysis of trended data
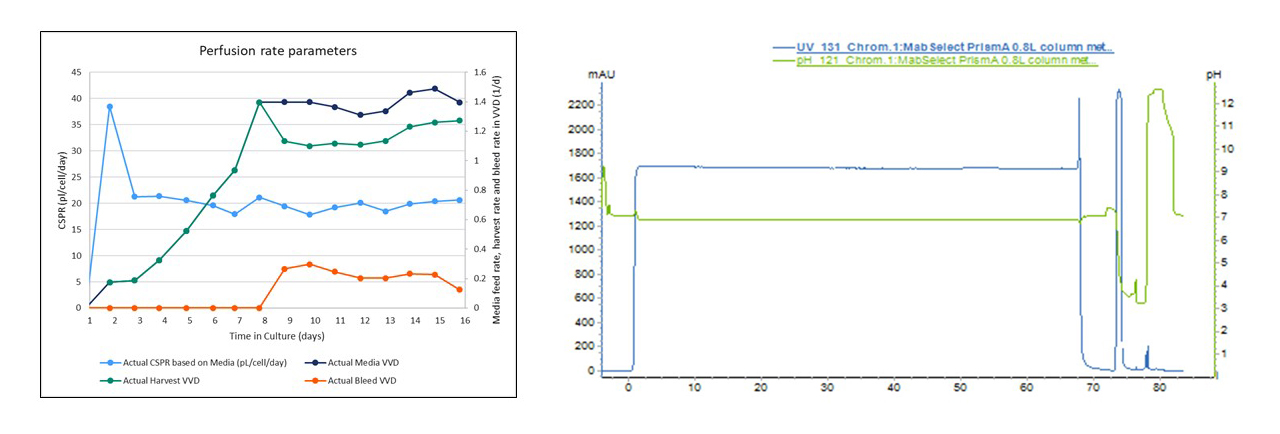
In a typical manufacturing process, each individual unit operation can be monitored on its own, however in connected processes, visualizing parallel unit trends should be considered.
Fig 6. Examples for process trend data from both upstream and downstream unit operations
Ideally key trends across unit operations should be identified and organized in accessible views. Example trends from small scale experiments to use as reference can also be helpful during the run. Because of the amount of samples and data generated, allowing for ample time and resources to perform a post-run analysis is also advised.
Post-run activities
After a run, it is important to clean the lab space, demount all flow kits, discard consumables and leftover liquids from upstream and downstream, and prepare the equipment for the next process. It is also important to know how to care for and store the product, i.e., the drug substance. Samples that will be analyzed also need to be handled correctly according to the sampling plan to ensure that errors are not introduced. After the process is completed it is also an opportunity to look for ways to improve the process as part of a continuous improvement exercise.
Conclusion
There are many advantages to using a continuous closed connected process instead of a traditional biomanufacturing process. These advantages include reducing equipment footprints, protecting the product from environmental contamination, and the need for less qualified cleanroom space. Development and execution of a closed connected single-use process requires however, a number of considerations with respect to risk mitigation and planning. Thorough planning, process simulations, and small-scale runs were identified as the most important activities to build for success in a closed connected single-use process.