What is a Fab fragment?
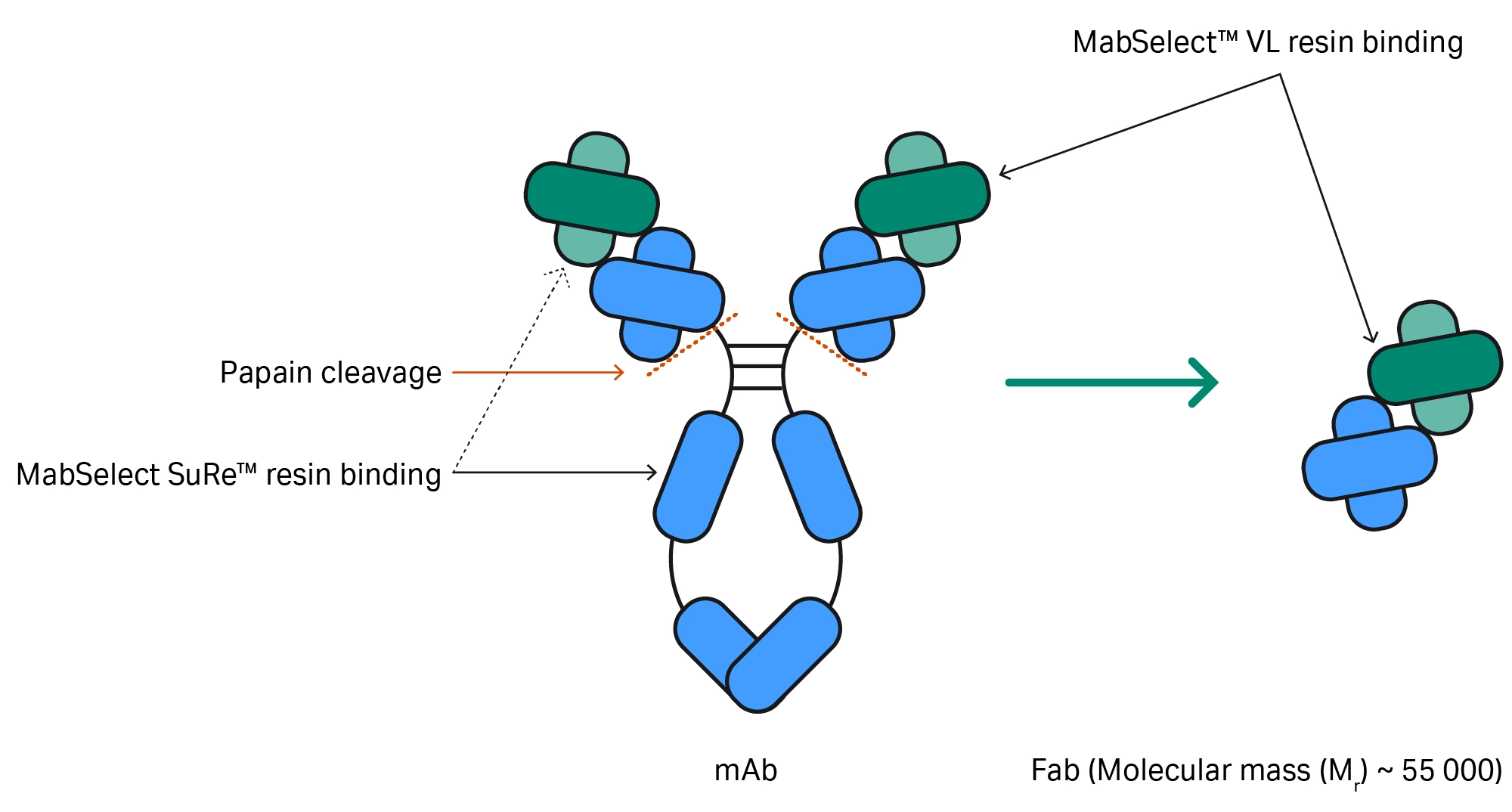
An antibody’s structure is Y-shaped, consisting of two Fab (fragment antigen binding) arms and a crystallizable fragment (Fc) tail (Fig 1). The Fab domains contain variable and constant regions made up of heavy and light chains, which bind to antigens. The Fc domain drives the molecule’s immune function.
Starting with an IgG antibody, individual Fab fragments are usually generated using the cysteine protease enzyme, papain, to cleave the antibody above the hinge region that connects the Fc and Fab domains. This process results in two Fab fragments and one Fc fragment.
Several biopharmaceuticals have Fab fragments as an active ingredient, using their specific binding capabilities for therapeutic purposes and avoiding any potential side effects from the full antibody. Purification of antibody fragments is therefore an important step for certain bioprocessing applications.
Affinity chromatography for antibody fragments
The use of affinity chromatography significantly simplifies biomolecule purification and enables the use of standard protocols. While antibodies can be purified using different affinity profiles and MabSelect™ resin options, selective purification of the Fab region requires added specificity. MabSelect™ VL protein L resin, specifically developed for applications targeting the variable light (VL) chain of an antibody, is an effective tool for the purification of Fab fragments.
Affinity purification of antibody-related proteins can be the only purification step in applications that do not require very high purity. When very high purity is needed (> 95%), a combination of different techniques can be used.
In the work presented here, we purified Fab fragments on ÄKTA go™ protein purification system using the Fc-binding capabilities of MabSelect SuRe™ antibody purification resin combined with the VL-binding of MabSelect™ VL protein L resin (Fig 1). ÄKTA go™ system is small and compact, so researchers can perform routine protein purification with ease while using bench and cold cabinet space efficiently (Fig 2). This process effectively removes antibody-related byproducts and other reagents from starting material derived from papain cleavage of a monoclonal antibody.
Fig 1. Following papain cleavage, MabSelect SuRe™ and MabSelect™ VL resins can be used to purify Fab fragments.
Fig 2. Preparing ÄKTA go™ protein purification system.
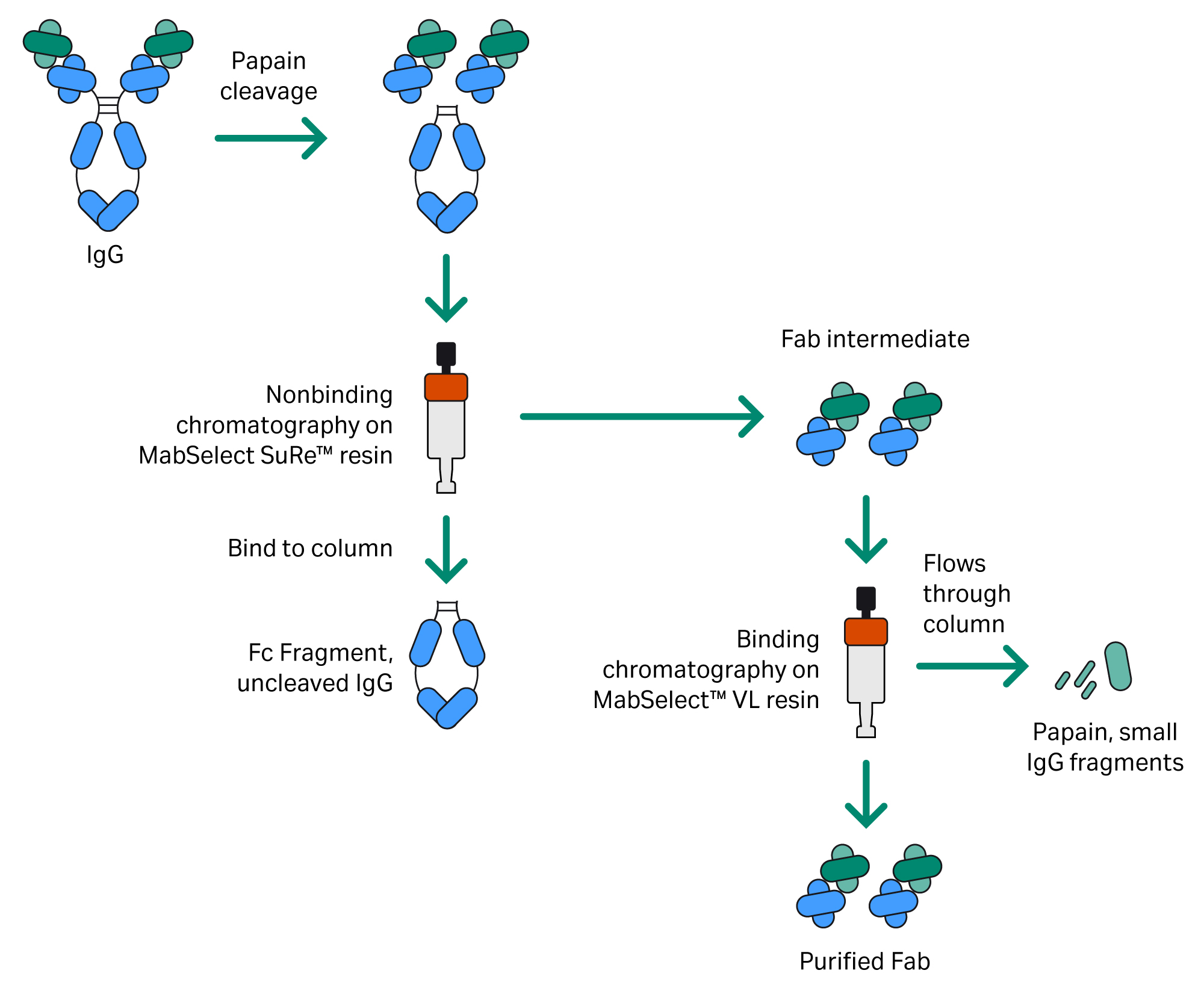
Fab fragments were purified from a papain digestion mixture using two chromatography steps (Fig 3). HiTrap™ prepacked chromatography columns for each step were connected to the system at the same time using the column valve (Fig 4).
Fig 3. Two-step Fab purification procedure using MabSelect SuRe™ and MabSelect™ VL resins.
Fig 4. The columns for each chromatography step were connected to the system using the column valve.
In the first step, the sample was applied to a 5 mL HiTrap™ column prepacked with MabSelect SuRe™ resin under conditions that favor only the binding of the Fc fragment and uncleaved antibody, while the Fab is retrieved in the unbound fraction.
Next, the unbound Fab fraction was applied to a 5 mL HiTrap™ column prepacked with MabSelect™ VL protein L resin under conditions that favor the binding of the Fab while allowing small fragments, papain, and other reagents to remain unbound. The purified Fab is then eluted from the column. Other formats, such as HiScreen™ or HiScale™ columns, can be used for purification, depending on the applied sample volume and the amount of Fab that is needed.
Predefined methods simplify purification
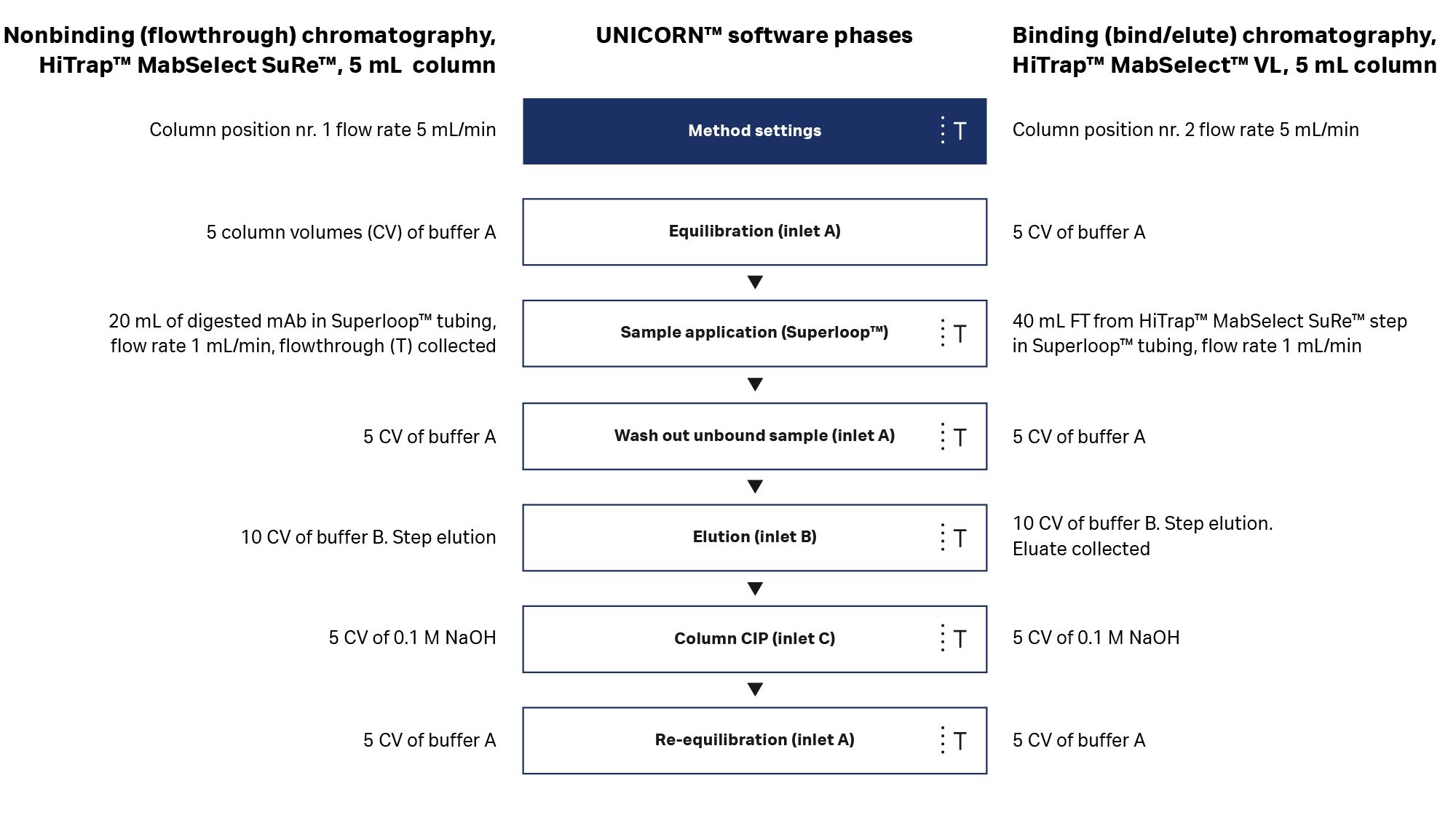
ÄKTA go™ system is fully supported by UNICORN™ software. With predefined methods, you can create an automated process for the most common chromatography techniques in minutes.
For this application, the process shown in Figure 5 was generated using predefined methods. The two purification steps were run using identical UNICORN™ methods, where the only difference to the programming was the chromatography column’s valve position. When the columns are already installed, the simple setup and programming of ÄKTA go™ system allow a fast (< 1 d) purification process. The second run requires only cleaning and loading of the Superloop™ sample application tube as well as hooking the system up to the buffers needed for the next step.
Fig 5. Slight modifications to the same predefined method for affinity chromatography were used for the purification steps. (mAb = monoclonal antibody; CIP = cleaning in place).
Results: Efficient and reliable Fab purification
Nonbinding (flowthrough) chromatography with MabSelect SuRe™ resin
Table 1. Protocol for the first step of the Fab purification with MabSelect SuRe™ resin prepacked in a 5 mL HiTrap™ column
| Stage | Column volume (CV) | Buffer | Flow rate (mL/min) |
| Equilibration | 5 | 50 mM citrate, 100 mM NaCl, pH 5.0 | 5 |
| Sample load | 5 | Digested mAb, pH adjusted to pH 4.9 | 1.25 |
| Wash | 5 | 50 mM citrate, 100 mM NaCl, pH 5.0 | 5 |
| Elution | 5 | 50 mM citrate, pH 3.5 | 5 |
| CIP | 5 | 0.1 M NaOH | 5 |
| Reequilibration | 5 | 50 mM citrate, 100 mM NaCl, pH 5.0 | 5 |
A 25 mL volume of papain-digested monoclonal antibody (mAb) was adjusted to a pH of 4.9 by addition of 1 M phosphate pH 2.0 and applied on the column through a Superloop™ sample application tube coupled to the sample valve of the ÄKTA go™ system.
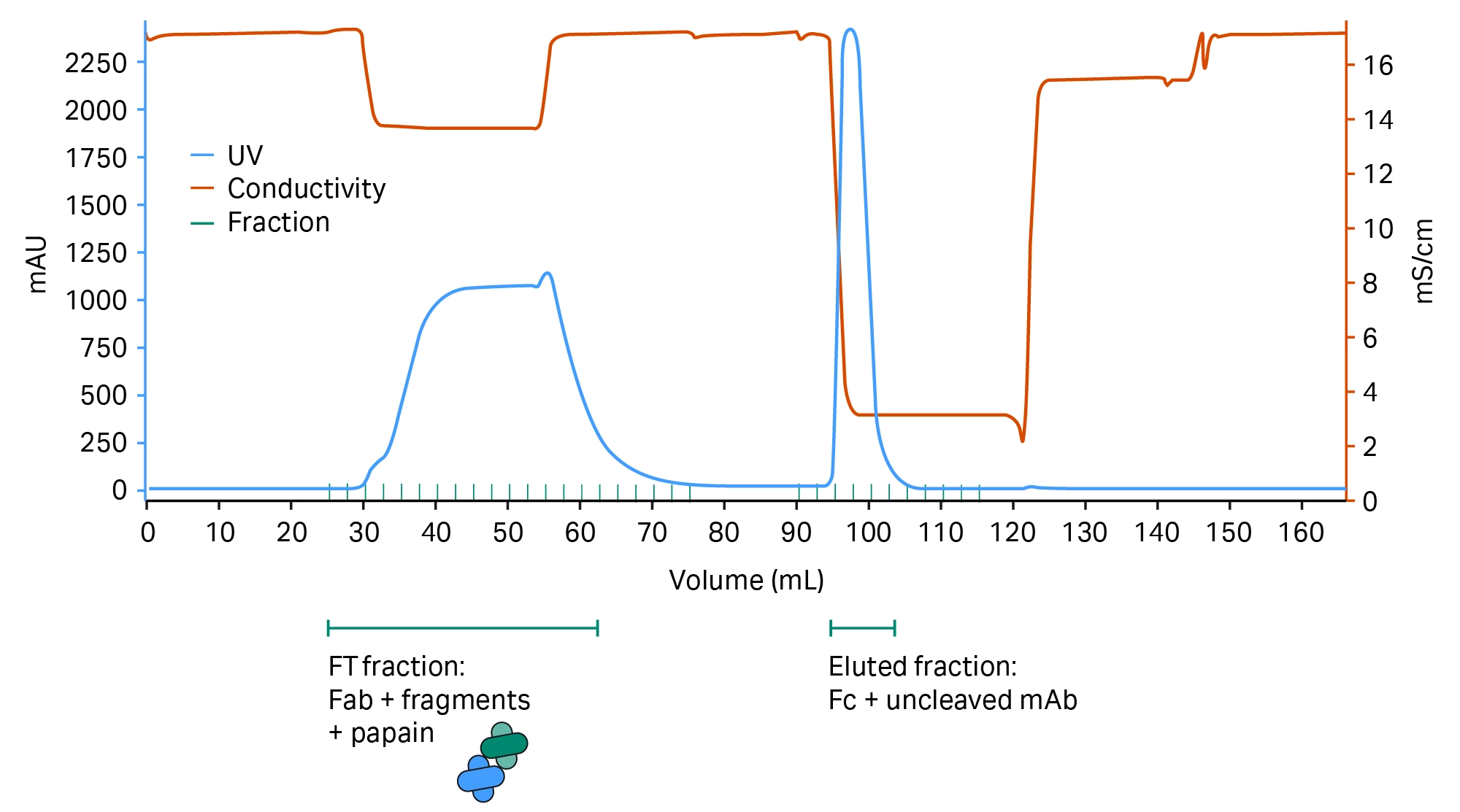
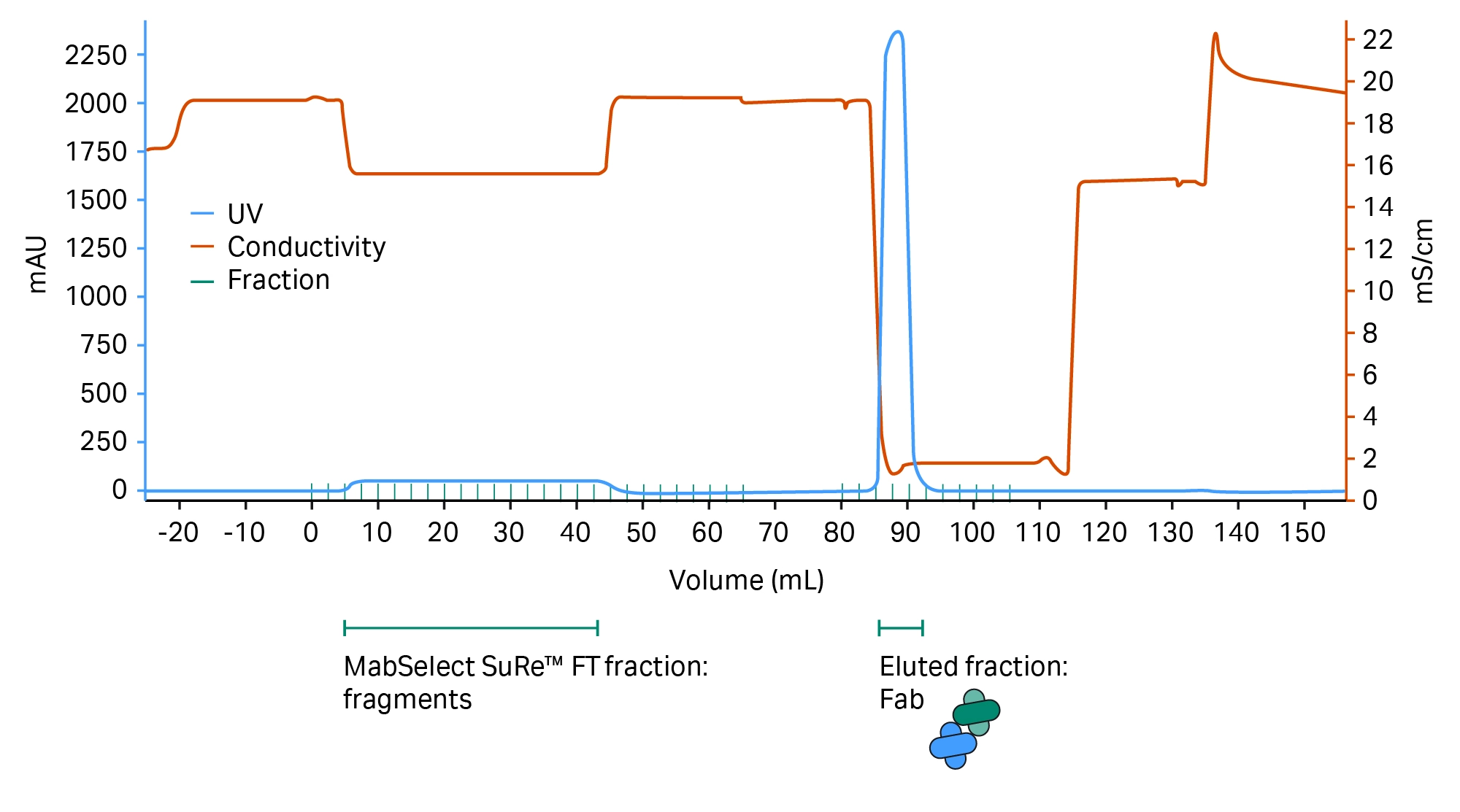
The pH of the equilibration buffer and sample is below the standard value for a MabSelect SuRe™ resin run (Table 1). This derives from the mAb having VH3-type subunits, where MabSelect SuRe™ resin shows a secondary affinity for the variable region on the Fab. The lower pH and the citrate buffer system both serve to diminish this affinity to ensure that the Fab remains in the flowthrough (FT) fraction and does not bind to the column.
Fig 6. Chromatogram showing MabSelect SuRe™ resin separation of the Fab and Fc parts of the antibody.
Separation of Fab and Fc parts of the antibody are shown in Figure 6.
Binding (bind and elute) chromatography using MabSelect™ VL resin
Table 2. Protocol for the second step of the Fab purification using MabSelect™ VL prepacked in a 5 mL HiTrap™ column
| Stage | Column volume (CV) | Buffer | Flow rate (mL/min) |
| Equilibration | 5 | 50 mM citrate, 100 mM NaCl, pH 6.8 | 5 |
| Sample load | 8 | FT fraction from MabSelect SuRe™ step, pH adjusted to pH 6.8 | 1.25 |
| Wash | 5 | 50 mM citrate, 100 mM NaCl, pH 6.8 | 5 |
| Elution | 5 | 50 mM citrate, pH 2.5 | 5 |
| CIP | 5 | 0.1 M NaOH | 5 |
| Reequilibration | 5 | 50 mM citrate, 100 mM NaCl, pH 6.8 | 5 |
The sample (FT from the MabSelect SuRe™ step) was adjusted to a pH of 6.8 to ensure binding of the Fab to the MabSelect™ VL ligand — 40 mL of sample was applied to HiTrap™ MabSelect™ VL column through a Superloop™ sample application tube (Table 2). The eluate fraction, containing the purified Fab, was immediately pH-adjusted to a pH of 5.4 after pooling to ensure protein stability.
Fig 7. The resulting MabSelect™ VL resin chromatogram shows how smaller fragments and reagents from the papain digestion flows through the column, while the Fab elutes in the low pH elution buffer in high concentration. The result is a Fab purity level that aligns with biopharmaceutical requirements.
The chromatogram showing the results from the purification using MabSelect™ VL resin is shown in Figure 7. Yields were calculated as A280 absorbance measured separately in the pooled fractions, given that the starting material was roughly 99% pure mAb and the amount of non-IgG related A280-absorbing reagents in the process was very low. The Fab fragments were assumed to constitute 66% of the total absorbance of the antibody.
Following these calculations, we received the following Fab yields:
- The nonbinding MabSelect SuRe™ chromatography step: 88%.
- The binding MabSelect™ VL chromatography step: 82%
- The final yield over the two-step purification would then be 72%
Size exclusion chromatography (SEC) results
Analysis of the fragments in the different intermediates was performed by SEC on a Superdex™ 200 Increase 10/300 GL column using PBS buffer at a flow rate of 0.8 mL/min (Fig 8).
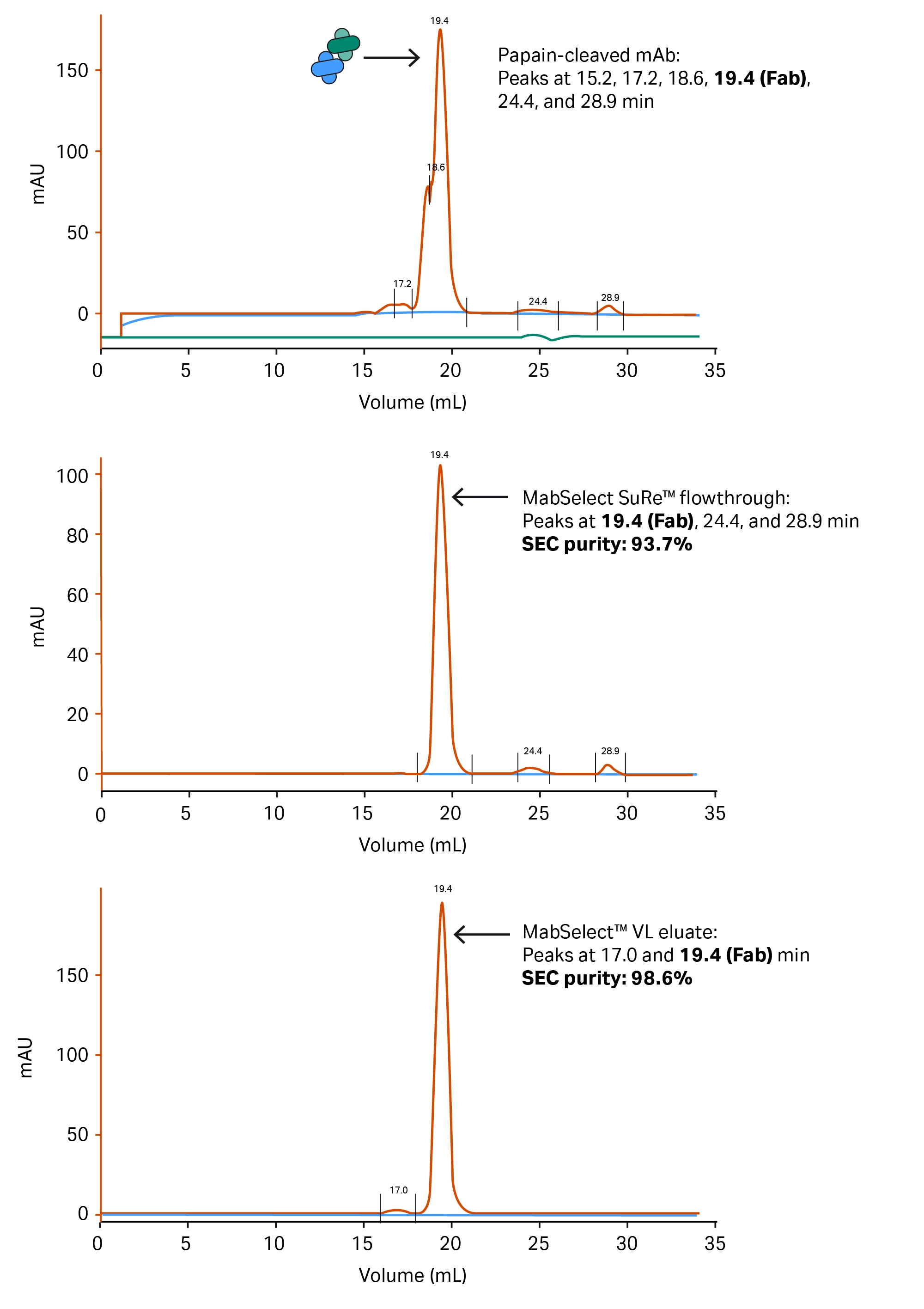
Fig 8. SEC chromatograms from the starting material (A), MabSelect SuRe™ flowthrough (B), and final material (C), showing the progression of purity throughout the procedure. The small peak at 17 min in the final material (C) is interpreted as a small amount of Fab dimer that was generated after the purification.
Electrophoresis results
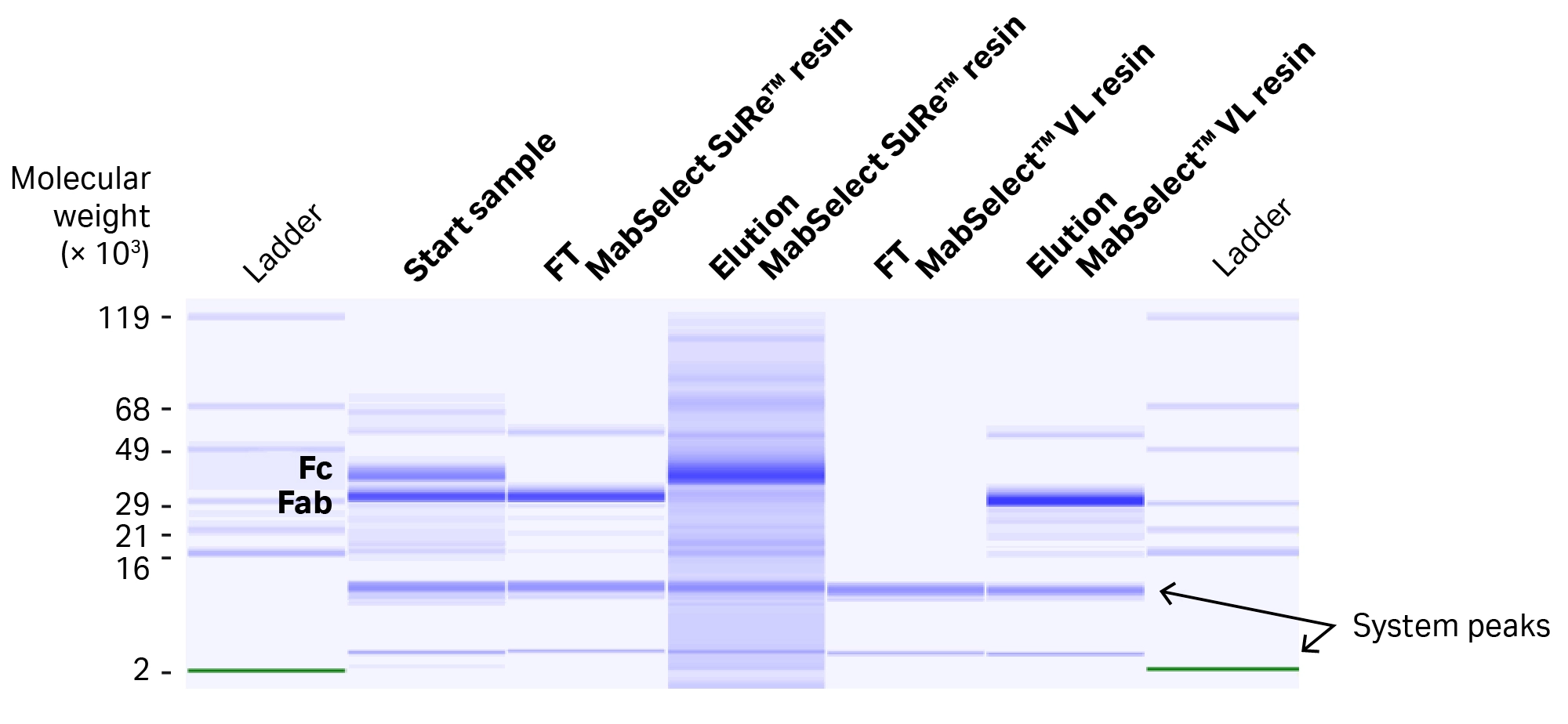
Identification of the different intermediates and results were further confirmed by electrophoresis on LabChip GXII Touch HT protein characterization system (Fig 9).
Fig 9. Electrophoretogram from the LabChip GXII Touch HT protein characterization system analysis, showing the composition of the protein material in the different intermediates in the purification procedure.
Summary and conclusions
- We have developed a two-step purification procedure for Fab fragments on ÄKTA go™ system using 5 mL HiTrap™ columns.
- The start material for the procedure is papain-digested mAb.
- The procedure first uses a 5 mL HiTrap™ MabSelect SuRe™ column to remove Fc fragments and other digestion products with affinity for the ligand.
- Adjustments with salt and pH were made in the MabSelect SuRe™ step to inhibit the secondary binding of ligand to VH3 domains in the Fab.
- The second step used a 5 mL HiTrap™ MabSelect™ VL 5 mL column to selectively bind the Fab fragments, further removing papain and small digestion products.
- The overall yield of the purification procedure was 72% as measured at 280 nm absorbance.
- The purity of the purified Fab was 98.6% according to SEC.
- The identity and purity of the intermediates and final product were confirmed by electrophoreses on the LabChip GXII Touch HT protein characterization system.
- The procedure uses reproducible and robust techniques and is run on ÄKTA go™ system in less than one day.
Click here for more information including additional applications, training, and tutorials for ÄKTA go™ protein purification system.
CY34819-01Jun23-WEB