Capture of bispecific antibodies and removal of product-related impurities using protein L resin
The biotherapeutics pipeline is becoming increasingly diverse as antibody variants such as bispecifics, conjugates, and fragments move through preclinical stages to commercial manufacturing. Many of these variants, especially asymmetric bispecific antibodies, are prone to aggregation or to forming product-related impurities such as homodimers and half antibodies during cell culture. The similarities between these impurities present extra challenges for downstream processes, especially post-capture. One potential solution is to initiate polishing at the capture step using differences in avidity. Here we present data on applications using MabSelect™ VL protein L resin with novel selectivity to purify challenging entities.
Introduction
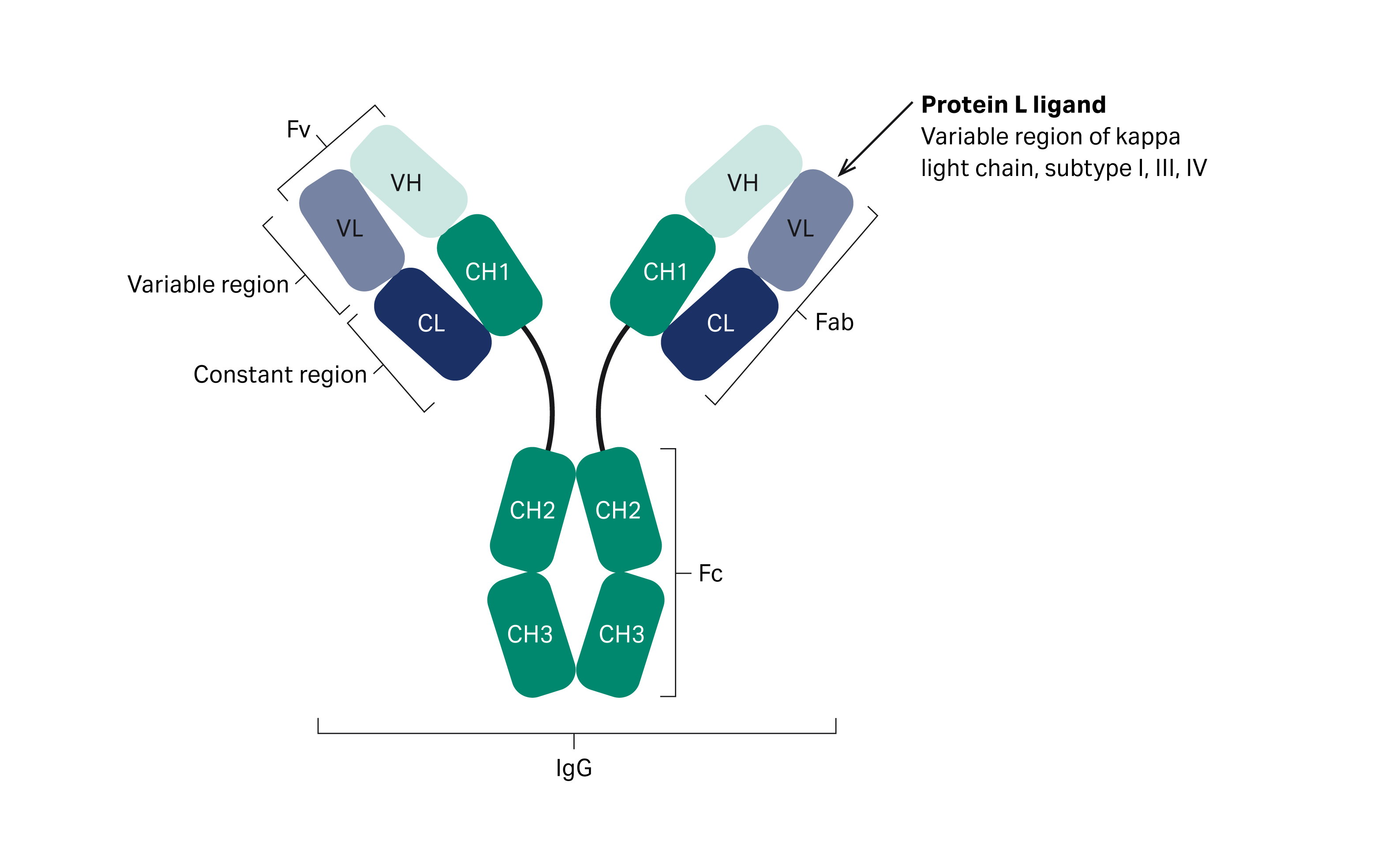
MabSelect™ VL affinity resin uses a protein L ligand with strong affinity for the variable region of the human antibody kappa light chain (Fig 1). The resin offers high productivity for affinity capture of bispecific antibodies (bsAb) and antibody fragments containing the kappa light chain. It also offers a good capture alternative for antibody variants that do not bind to protein A. This resin has substantially improved dynamic binding capacity (DBC) and alkaline stability compared to its predecessor, making it well suited for cost-efficient capture of antibody variants. MabSelect™ VL resin allows for good resolution of product-related impurities in the capture of bispecific antibodies, and it provides a tool for efficient purification of antibody variants to high purity. Here we present a typical application for separation of bispecific antibodies from mispaired homodimers.
Antibody structure
Fig 1.The structure of an antibody. The arrow indicates where protein L interacts with the antibody.
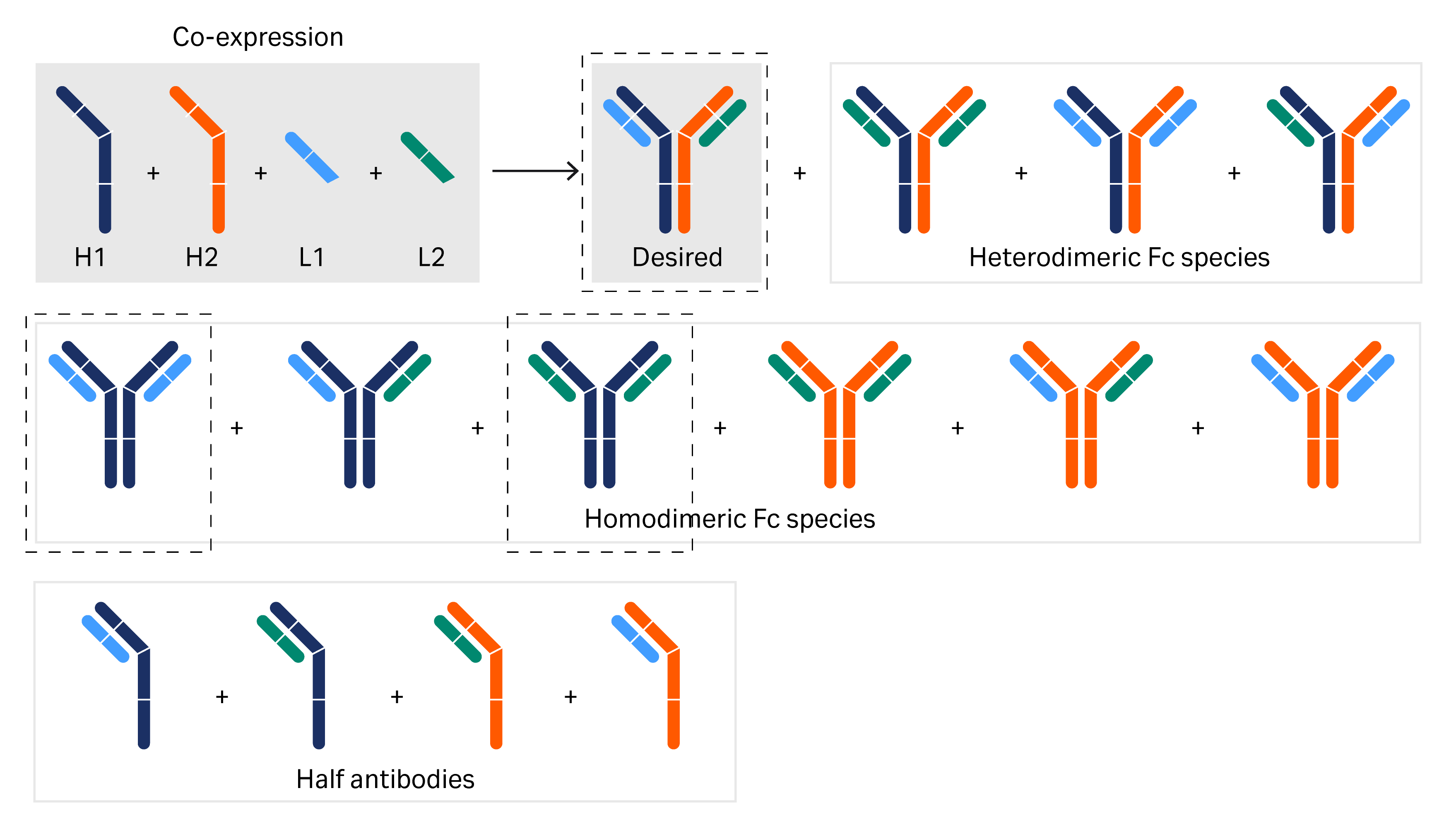
A bsAb with four chains can have various combinations of the heavy- and light-chain regions, as well as half antibodies that represent the desired bsAb and product related impurities (Fig 2). The type and amount of product-related impurities depend on the assembly technique, such as Fc heterodimerization used to produce asymmetric molecules, Knobs-into-holes technology, CrossMAb™ technology , or light-chain method used for correct pairing of light and heavy chains.
Fig 2. Possible combinations of the heavy- and light-chain regions in the expression of a bispecific mAb. The desired bispecific molecule and the mispaired homodimers are separated in the example described below.
pH elution for separation of bsAb and mispaired homodimers
MabSelect™ VL chromatography resin is designed to bind the kappa class light chains of IgG monoclonal antibody variants and can be used to separate the target molecule from mispaired versions in the capture step. We evaluated a separation based on kappa and lambda light chains and on differences in avidity using a feed composition comprised of a kappa-light chain (Trastuzumab kappa class 1 Anti-HER2 light chain), lambda light chain (Avelumab lambda class 2 Anti PDL1 light chain), and a FC chain (Anti-HER2 heavy chain 1 and 2) (Thermo Fisher Scientific). The different chains were targeted to express in a ratio of 30:30:40 respectively.
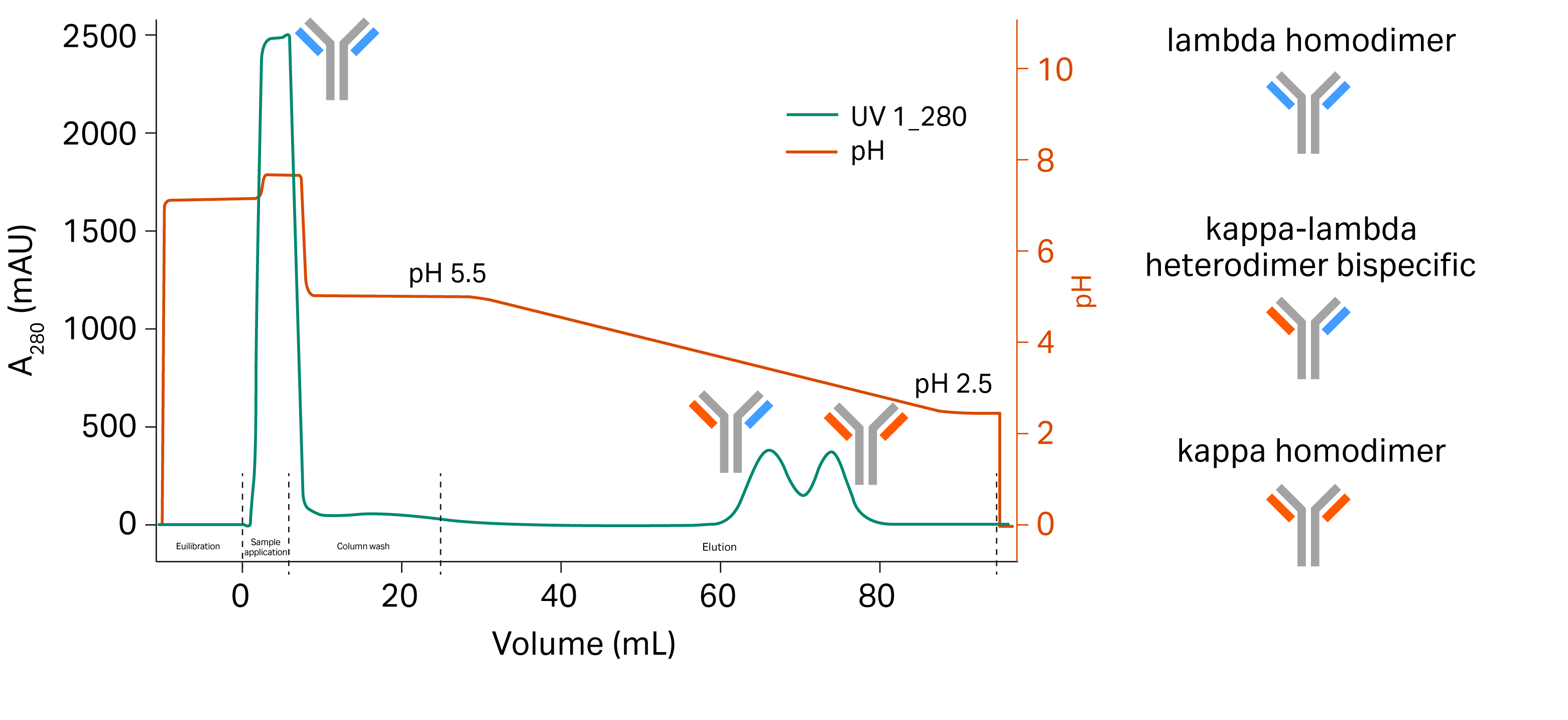
Initially, the elution pH for the bispecific kappa-lambda heterodimer and the mispaired kappa homodimer was determined with a gradient elution from pH 5 to pH 2.5 by loading 5 mg of the bispecific cell culture on a Tricorn™ 5/100 column packed with MabSelect™ VL resin (Fig 3). The fractions were collected during the elution gradients and analyzed with liquid chromatography–mass spectrometry (LC-MS) to confirm the separation of the different entities. Mispaired lambda homodimers do not bind to the column as they do not contain any kappa light chains and can be found in the flowthrough. The gradient elution from pH 5 to pH 2.5 separates mispaired kappa homodimer and the kappa-lambda heterodimer bispecifics due to differences in avidity.
Fig 3. Gradient pH elution separates the desired bsAb from the mispaired homodimers.
To further show the performance of MabSelect™ VL in separation of the bispecific kappa-lambda light chain mAb heterodimers from mispaired homodimers in cell culture harvest, the efficacy of a stepwise pH elution was tested. Step elutions were performed for different column formats packed with MabSelect™ VL resin. 5 mg of bispecific cell culture harvest was applied to a Tricorn™ 5/100 column, 2.5 mg was applied to a HiTrap™ 1 mL column, and 12 mg sample was applied to a HiScreen™ column.
Results
Gradient elution of bsAb sample
A linear gradient elution was performed using MabSelect™ VL resin packed in Tricorn™ 5/100 column to find the elution pH to be used in the step gradient runs (Fig 4). The results are presented in Table 2. The elution pH difference for MabSelect™ VL between the two peaks was 0.4.
Column: Tricorn™ 5/100
Resin: MabSelect™ VL
Sample: 5 mL of bsAb at a flow rate of 0.250 mL/min via a Superloop™
Equilibration: 20 mM Na-phosphate, 150 mM NaCl, pH 7.2
Wash: 50 mM citrate pH 5.0, 10 CV at 1 mL/min
Gradient: 0 to 100% of 50 mM citrate, pH 2.5 for 20 CV at a flow rate of 1 mL/min per step, followed by 100% for 5 CV
CIP: 3 CV of 0.1M NaOH at 0.2 mL/min
System: ÄKTA pure™
Fig 4. Chromatogram of linear gradient elution performed using MabSelect™ VL resin packed in Tricorn™ 5/100 column on ÄKTA pure™ chromatography system.
A step elution was then performed according to the elution pH obtained from the gradient elution (Table 1). 50 mM citrate pH 3.5 and pH 3.1 were used for elution steps 1 and 2, respectively.
Table 1. Elution pH found in the gradient elution pH 5 to 2.5. These values will be used as step elution settings (%B) with elution buffer 50 mM Citrate pH 2.5.
| Resin | Column | Elution pH peak1 | %B peak 1 | Elution pH peak 2 | %B peak 2 |
| MabSelect™ VL | Tricorn™ 5/100 | 3.5 | 65 | 3.1 | 79 |
Figure 5 presents the MabSelect™ VL step elution with different column formats. The results show similar elution profiles for Tricorn™ 5/100 and HiScreen™ columns. The elution peaks for HiTrap™ 1 mL were slightly delayed and broader compared to the other two columns. This is expected due to the smaller resin volume relative the void volume of the ÄKTA pure™ system compared to the other column formats. The relative larger void volume compared to the volume of HiTrap™ 1 mL causes dilution of the sample. All three column formats resulted in separation of the bsAb and the kappa homodimer.
(A)
Column: Tricorn™ 5/100
Resin: MabSelect™ VL
Sample: 5 mg of bsAb cell culture harvest
Equilibration: 5 CV of 20 mM Na-phosphate, 150 mM NaCl, pH 7.2 at 1 mL/min
Wash: 10 CV of 50 mM citrate, pH 5.0 at a flow rate of 1 mL/min
Elution: step gradient with 50 mM citrate buffer, pH 3.5 and pH 3.1at 1 mL/min
CIP: 3 CV of 0.1 M NaOH at 0.2 mL/min
System: ÄKTA pure™
(B)
Column: HiTrap™ column (1 mL)
Sample: 2.5 mg of bsAb cell culture harvest
Equilibration: 5 CV of 20 mM Na-phosphate, 150 mM NaCl, pH 7.2 at 0.5 mL/min
Wash: 10 CV of 50 mM citrate, pH 5.0 at 0.5 mL/min
Elution: step gradient with 50 mM citrate buffer, pH 3.5 and pH 3.1 at 0.5 mL/min
CIP: 3 CV of 0.1 M NaOH at 0.1 mL/min
System: ÄKTA pure™
(C)
Column: HiScreen™ column
Sample: 12 mg bsAb cell culture harvest
Equilibration: 5 CV of 20 mM Na-phosphate, 150 mM NaCl, pH 7.2 at 2.3 mL/min
Wash: 10 CV of 50 mM citrate, pH 5.0 at 2.3 mL/min
Elution: step gradient with 50 mM citrate buffer, pH 3.5 and pH 3.1 at 2.3 mL/min
CIP: 3 CV of 0.1 M NaOH at 0.47 mL/min
System: ÄKTA pure™
Fig 5. Chromatogram showing purification of bsAb sample using MabSelect™ VL resin packed in (A) Tricorn™ 5/100 (dark blue), (B) HiTrap™ 1 mL (light blue), and (C) HiScreen™ (green) columns and eluted in a step gradient with 50 mM citrate buffer, pH 3.5 and pH 3.1.
LC-MS analysis of elution fractions
To confirm the separation of product-related impurities such as homodimers and half-antibodies the fractions were analyzed with LC-MS.
The samples used in the gradient and step elution runs were treated before LC-MS analysis with FabRICATOR® (IdeS, Genovis AB). Fc/2 fragment were present in all fractions and corresponded to the peak molecular weight (Mr) of approximately 25 000 (Fig 6). Fraction A12 was composed of a main peak of approximately Mr 97 000 which matched the MW of the bsAb. Fraction B3 contained the bsAb (Mr 96 970) and the kappa homodimer (Mr 97 600) but also smaller fragments (Mr 23 600 and 46 200) which are likely variable light change (VL) and dimerized VL. The remaining fractions (B4, B5, and B6) contained only the kappa homodimer and no bsAb. Fragments, such as VL and dimerized VL, were present in fractions B4 and B6, respectively.
The results show that the mispaired kappa homodimer and the kappa-lambda heterodimer bispecific mAb are separated well which can be seen in the Mr of 97000 and 97600 separation in LC-MS from the different elution fractions. The data also shows that the bsAb can not only be purified from the kappa homodimer but also separated from the single and dimerized VL in the sample.
Fig 6. LC-MS data on fractions from gradient elution of bsAb sample using MabSelect™ VL resin
The LC-MS analysis performed on the fractions from the step elution shown in Figure 7 are presented in Figures 8 and 9. The step elution marks a baseline separation of the mispaired kappa homodimer and the kappa-lambda heterodimer bsAb. The LC-MS data from the gradient and step elutions show a clear separation of the bsAb from the impurities such as mispared kappa homodimer and single and dimerized VL.
Column: Tricorn™ 5/100
Resin: MabSelect™ VL
Sample: bsAb
Sample load: 5 mg
Start buffer: 20 mM Na-phosphate, 150 mM NaCl, pH 7.2
Wash: 50 mM citrate, pH 5.0
Elution buffer: 50 mM citrate buffer
Flow rate: 1 mL/min
Gradient: step gradient elution, pH 3.5 and pH 3.1
System: ÄKTA pure™
Detection: UV 280
Fig 7. Chromatogram of step elution with fraction IDs of bsAb using MabSelect™ VL chromatography resin in Tricorn™ 5/100.
Fig 8. LC-MS data of fraction A5 from step elution showing MW of Fc/2 (Mr 25 000) and kappa-lambda heterodimer bsAb (Mr 97 000).
Fig 9. LC-MS data of fraction C2 from step elution showing MW of Fc/2 (Mr 25 000) and mispaired kappa homodimer (Mr 97 600).
Conclusions
We have shown that:
- MabSelect™ VL can separate kappa-lambda heterodimer bsAb from impurities (kappa homodimer and VL) due to differences in avidity. The desired bsAb was collected in the first elution peak over a gradient pH elution. Since the kappa homodimers and VL domain bind stronger to the resin, they eluted later in the second peak.
- A step elution with two static pHs resulted in a baseline separation of the kappa-lambda heterodimer bsAb and the impurities in the cell culture harvest.
- The resolution in a step pH elution with MabSelect™ VL is comparable for the different column formats (Tricorn™ 5/100, HiTrap™ 1 mL, and HiScreen™) investigated in this study. The elution peaks for HiTrap™ 1 mL were slightly delayed and broader compared to the other two columns. This is expected due to the smaller resin volume relative the void volume of the ÄKTA pure™ system compared to the other column formats. All three column formats result in baseline separation of the bsAb and the kappa homodimer.
Material and methods
A linear gradient elution was performed using MabSelect™ VL resin packed in Tricorn™ 5/100 column to find the optimal elution pH. Next, step elutions were performed for different column formats packed with MabSelect™ VL resin to compare performance (Table 4). The buffers used in the gradient and step elution are listed in Table 3. ÄKTA pure™ chromatography system with preprogramed UNICORN™ software methods were used for both the linear gradient and step elution runs.
Table 3. Buffers used in the gradient and step elution.
| Buffer | Composition |
| A1 (equilibration buffer) | 20 mM Na-phosphate, 150 mM NaCl, pH 7.2 |
| A2 (wash buffer) | 50 mM citrate, pH 5.0 |
| B1 (elution buffer) | 50 mM citrate, pH 2.5 |
| A3 (CIP) | 100 mM NaOH |
Table 4. Elution setup for step elution based on the results from the gradient elution
| Column | %B step 1 | CV | %B step 2 | CV |
| Tricorn™ 5/100 column (2 mL) | 65 | 8 | 79 | 5 |
| HiTrap™ column (1 mL) | 65 | 8 | 79 | 8 |
| HiScreen™ (4.7 mL) | 65 | 8 | 79 | 8 |
LC-MS analysis of elution fraction
To further confirm the separation of product-related impurities such as homodimers and half-antibodies in a step elution, the fractions under peak 1 and peak 2 of the step elution using MabSelect™ VL were analyzed with LC-MS to determine the masses of the different entities in the elution pools. 5 mg bsAb sample was applied to MabSelect™ VL in Tricorn™ 5/100 and a step gradient elution was performed including strip (100 mM Citric acid pH 2.1) and Milli-Q wash before CIP with 100 mM NaOH. Selected fractions were analyzed with LC-MS.
The samples were treated with FabRICATOR® (IdeS, Genovis AB) prior to LC-MS analysis to digest bsAb samples. Samples were incubated for 3 hr with the enzyme and analyzed using LC-MS. Reverse phase chromatography with gradient of 0.1% formic acid and 0.1% formic acid in acetonitrile solvents as buffers were used prior to MS as a separation technique. Using high accuracy mass spectrometry enabled the detection of homo and heterodimers along with small fragment molecular weights.