We have successfully designed a closed and physically connected mAb process at the pilot scale with single-use components. This article describes the purification process from a 50 L perfusion culture.
- The purification process was divided into two separate processing trains. One process used a prepacked ReadyToProcess™ MabSelect PrismA™ column for the capture step. The second process used a pilot-scale Fibro™ PrismA unit, a fiber based technology, which enables single-use rapid cycling chromatography.
- When comparing the final products from the two separate process trains it can be concluded that the processes were comparable and produced materials for both process trains had a high yield and high quality, with a processing time advantage for Fibro PrismA in the capture step.
- The use of sterile ReadyToProcess™ articles connected with ReadyMate™ connectors to build the closed process line was successful. No bioburden was detected in the majority of the analyzed intermediates and endotoxin levels were below detection limit for all samples collected downstream of the harvest tank.
Introduction
Pharmaceutical manufacturers are striving for more diversified portfolios targeting a greater number of monoclonal antibodies (mAb), the key to driving down overall costs is to lower capital expenditure (CAPEX) investments by better utilization of equipment. This drives the need for flexible solutions that can reduce facility footprint and minimize changeover time between campaigns.
Strategies to address these demands include the use of variable degrees of continuous processes, which enables the use of smaller chromatography columns and bioreactors. This, in turn, allows for reduced buffer consumption and overall facility footprint. Complementary approaches also include designing closed and connected process steps using single-use equipment and consumables to enhance flexibility and minimize the need for expensive clean rooms and storage space.
The purpose of the described study was to develop a closed and physically connected downstream purification process for material from a perfusion cell culture. The equipment and consumables were primarily single-use and designed for a manufacturing process in a good manufacturing practices (GMP) environment. To compare the performance of resin based chromatography to fiber-based chromatography methods, the purification process was divided into two separate processing trains. One process used a prepacked ReadyToProcess MabSelect PrismA column for the capture step. The second process used a pilot-scale Fibro PrismA, a fiber-based technology that enables single-use, rapid cycling chromatography. The Fibro PrismA unit used in this study was a prototype 160 mL unit and is not yet commercially available.
Downstream process design
The material to be purified was harvested from a 50 L perfusion cell culture, which was run continuously for 10 days. The average daily volume was 70 L, and average titer was 0.64 g/L but varied slightly over the process time. Learn more about the upstream perfusion culture process here.
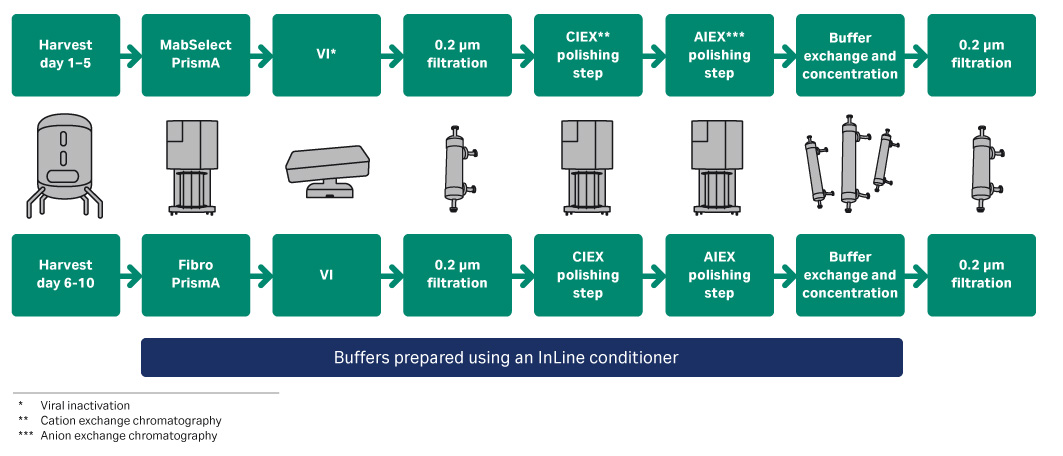
Purification was performed using a standard three-step mAb purification process. The downstream process was divided into two separate processing trains (Fig 1), with the main difference being the use of ReadyToProcess MabSelect PrismA or Fibro PrismA for the capture step. All buffers for the process, except for acid and base solutions used for the viral inactivation step, were prepared from stock solutions using an in-line conditioning (IC) system.
Fig 1. Process flow chart for the connected mAb purification processes.
Clarified harvest was continuously collected in an Xcellerex™ XDUO-200 bioreactor from day 7 of the perfusion bioreactor cultivation at the time point when cell density steady state was reached. At cultivation day 8 the collected harvest was loaded onto a ReadyToProcess MabSelect PrismA 0.8 L column connected to an ÄKTA ready™ system with low flow kit as one sub-batch. This was then repeated daily for 5 consecutive days resulting in 5 captured sub-batches (batch 1 through 5). Column load in each sub-batch was between 57 to 59 g antibody/L resin corresponding to approximately 90% of the evaluated QB10.
Then, for days 6 through 10 (bioreactor day 13 through 17) the harvest material was processed using a 160 mL Fibro PrismA unit. The load in each cycle was approximately 3.5 g antibody (~ 22 g/L matrix load). The eluate peak was collected starting 1.5 matrix volumes after elution step started and ended when UV280 decreased to 200 mAu on the downslope of the eluate peak.
Following the capture step, low pH viral inactivation of the eluted product was performed in a WAVE™ 25 rocking bioreactor. The inactivated material was 0.2 µm filtered and stored until the next step. Pooling of material was performed after the viral inactivation step to achieve the proper loading on the polishing steps.
For both process trains, ÄKTA ready with ReadyToProcess™ Capto™ S Impact column in bind-elute mode was used as the first polishing step followed by a ReadyToProcess Q Adsorber Membrane in flow through mode as the final polishing step. ÄKTA readyflux™ system with ReadyToProcess hollow fiber cartridges for UF/DF was used to concentrate the final purified product.
Materials and methods
Detailed materials and methods are available at the end of the page.
Results
Overall process performance
Process design using single-use equipment and consumables to enable a closed, aseptic, and connected process worked very well. No viable microorganisms were detected in the majority of the analyzed intermediates and endotoxin levels were below detection limit for all samples collected. In two of the samples where 1 CFU was reported, duplicate samples were negative. Positive results were most likely attributed to sampling or testing errors and not confirmed with endotoxin results or a second sample.
The overall process yields and purification performance for the two process trains were comparable (Table 1).
In the selected process design where perfusion harvest material was processed daily in a batchwise manner, Fibro PrismA chromatography resulted in substantially reduced process time for the capture step compared with MabSelect PrismA.
Table 1. Summary of overall process performance results
| Data type | MabSelect PrismA process train |
Fibro PrismA process train |
| HCP (ppm) | 8 | 9 |
| DNA (ng/mg) | < 0.026* | < 0.026* |
| HMW (%) | 1.6 | 1.5 |
| Residual protein A leakage level (ppm) | < 0.03 | < 0.03 |
| Total process yield (%) | 82 | 80 |
| Bioburden (CFU/mL) | 0 | 0-1 |
| Endotoxin (EU/mL) | < 0.5 | < 0.5 |
| Process time (Capture step) | 7.7 h (1 cycle per day) | 3.5 h (10 cycles per day) |
*Below limit of quantification of analytical method.
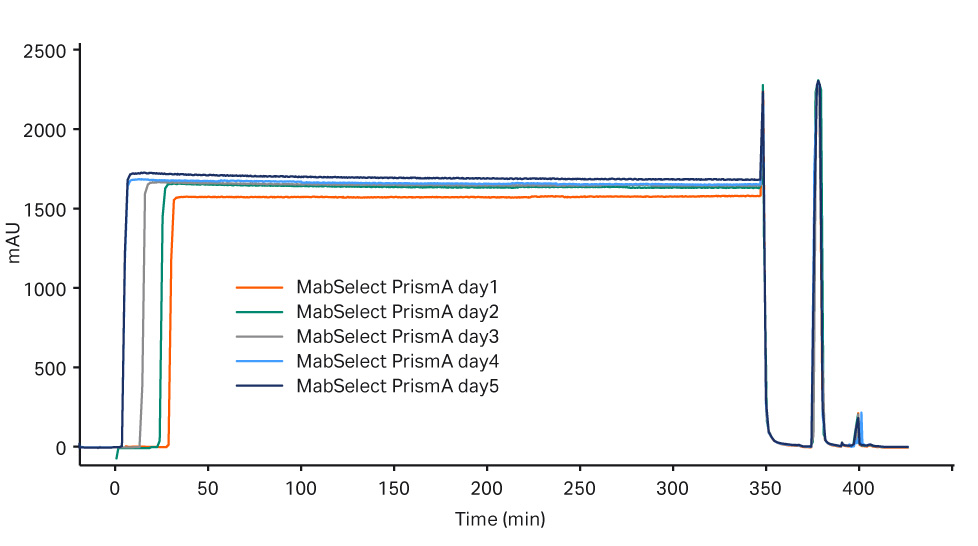
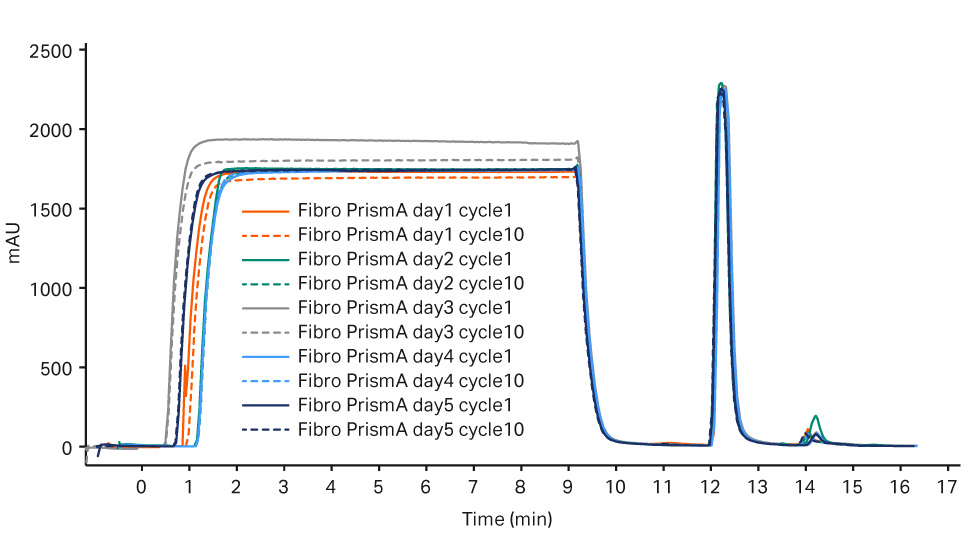
Purification performance over five days for each process train was consistent for the capture step, see Figure 2 for MabSelect PrismA and Figure 3 for Fibro PrismA results.
Fig 2. Overlay of chromatograms for day 1-5 for the MabSelect PrismA step.
Fig 3. Overlay of chromatograms for day 6-10 for the Fibro PrismA step.
Process yield
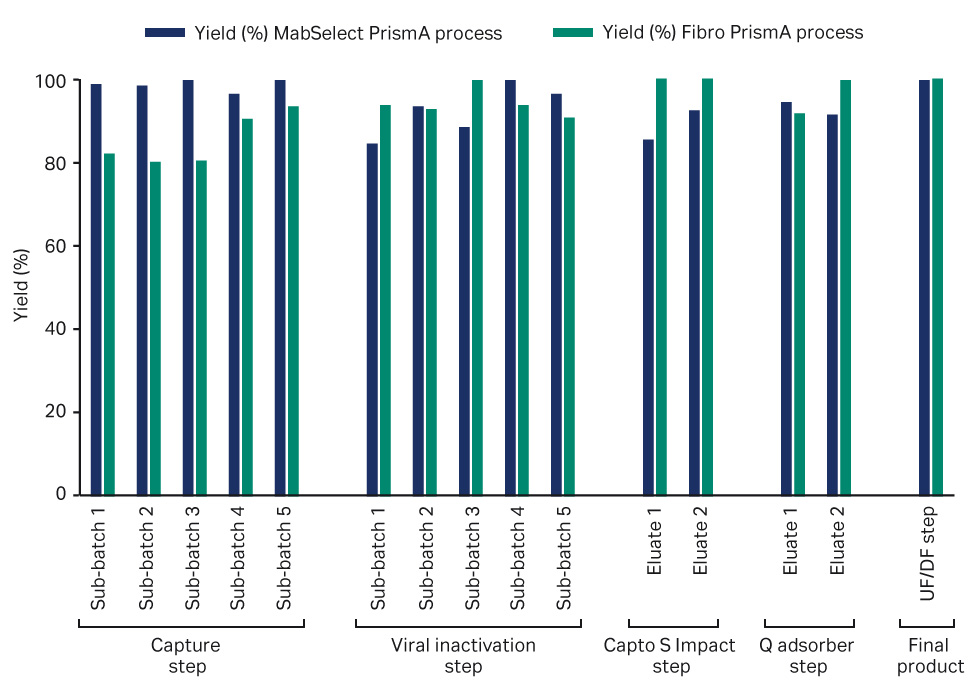
The product recovery across the unit operations for the two process trains can be seen in Figure 4. Total process yield is comparable for both process trains.
The yield of the MabSelect PrismA column over the capture step was consistently high over all the sub-batches. The yield of the PrismA Fibro unit varied between sub-batches with a mean recovery over the five batches of 85%. The lowest recovery was seen in the first three sub-batches and the last two sub-batches were above 90%. Further optimization of the elution step needs to be performed due to the fast process cycling time. Yield can be improved using adapted and optimized collection criteria from the ÄKTA ready system.
Fig 4. Product recovery over the separate unit operations. Column sub-batches blue and Fibro sub-batches green.
The yield over the viral inactivation step for the MabSelect PrismA process train was lower in the first two batches and higher in the final three batches. The larger volume losses in the first two batches were likely the result of using a 0.2 µm ULTA™ HC filter with greater surface area for the filtration step. The yield for the Fibro PrismA process train over the viral inactivation step was consistently high across all sub-batches.
Conclusion
The aim of this study was to develop a closed, aseptic, and connected mAb purification process for a GMP environment using single use equipment and consumables. The downstream process was connected to a 50 L bioreactor in perfusion mode. The results showed that a successful closed and connected downstream process was achieved. There were no issues with bioburden or endotoxins throughout the process and over time.
The results also showed comparable performance for the two examined process trains. One with a high capacity protein A resin, MabSelect PrismA, and the other one with Fibro PrismA, a rapid cycling, single-use chromatography technology. The chromatography resin enables higher binding capacity while the Fibro units enable faster processing times or alternatively smaller adsorber volumes. As these two technologies have different benefits depending on the desired outcome, this offers enhanced options to design intensified mAb purification processes based on existing facility design, design constraints, and desired process outcome.
Materials and methods
Buffer preparation
All buffers for the process except for acid and base for the viral inactivation were prepared from HyClone™ stock solutions using an IC system. The buffers were mixed by the system and 0.2 µm filtered into sterile ReadyToProcess storage bags before use in the process, i.e., the filter was connected to the outlet of the system.
System and column preparation
The ÄKTA ready chromatography system was prepared prior to process start by connecting flow kit installation solutions, buffers and product collection bags aseptically through ReadyMate single-use connectors to the ÄKTA ready flow kit. Y-manifolds with ReadyMates were used to extend the number of inlets on the flow kit. The column was connected to the flow kit through TC-connectors in a sterile bench prior mounting of the assembly into the ÄKTA ready system. After installation a CIP of the flowpath and column was done. The system setup was then used for 5 sub-batches on the MabSelect PrismA capture step and the same procedure repeated for the Fibro PrismA step. The ÄKTA ready systems was prepared in the same way for the polishing steps.
MabSelect PrismA capture and viral inactivation step
Clarified harvest was continuously collected in a XDUO-200 from day seven of the perfusion bioreactor cultivation when steady-state cell density was reached. At cultivation day eight the collected harvest was loaded onto a ReadyToProcess MabSelect PrismA 0.8 L column as one sub-batch. This was then repeated daily for five consecutive days resulting in five captured sub-batches (batch 1-5). Column load in each sub-batch was between 57 to 59 g antibody/L resin corresponding to approximately 90% of the evaluated QB10. The chromatography method is shown in Table 2. The eluate peak was collected from a UV280 rise at 100 mAu and ended when UV280 decreased to 100 mAu on the downslope of the eluate peak
Table 2. ReadyToProcess MabSelect PrismA chromatography method
| Description | Buffer | CV | Flow rate (residence time) |
| Equilibration | 20 mM NaP, pH 7.0 + 0.5 M NaCl | 5 | 4 min |
| Load | Clarified harvest mAb8 | n/a | 4 min |
| Wash 1 | 20 mM NaP, pH 7.0 + 0.5 M NaCl | 5 | 4 min |
| Wash 2 | 50 mM Na-acetate, pH 5.5 | 2 | 4 min |
| Elution | 50 mM Na-acetate, pH 3.5 | 3 | 4 min |
| Strip | 0.1 M HAc | 2 | 4 min |
| CIP | 0.5 M NaOH | 3 | 4 min |
| Re-eq | 20 mM NaP, pH 7.0 + 0.5 M NaCl | 5 | 4 min |
| Storage | 20% EtOH | 5 | 4 min |
Each daily eluted product pool was collected in a 2 L WAVE cell bag for viral inactivation. The eluate was adjusted to pH 3.5 ± 0.1 by addition of acid. The adjusted eluate was incubated with gentle rocking for 60 to 75 minutes and then readjusted to pH 5.0 by addition of 2M Tris. After the viral inactivation step the material was 0.2 µm filtered using a ULTA Pure HC 0.6/0.2 µm 4" Capsule Filter for sub-batch 1 and 2 and a smaller 2" filter for sub-batch 3 to 5 and stored at 2-8°C until performance of the Capto S ImpAct step. No pressure increase was observed for either of the filtrations.
Fibro PrismA capture step and viral inactivation
On purification day six (bioreactor day 13) the ÄKTA ready flow kit and the chromatography column was exchanged for a Fibro PrismA 160 mL unit to be able to evaluate and compare the Fibro format to the column format. From this time point the collected harvest was loaded onto the Fibro unit as 10 cycles every day, which was pooled to one sub-batch before viral inactivation. This was repeated daily for five consecutive days resulting in five captured sub-batches (batch 6-10). Fibro load in each cycle was approximately 3.5 g antibody (approximately 22 g/L matrix load). The chromatography method is shown in Table 3. The eluate peak was collected from a set volume of 1.5 matrix volumes into the elution block to an UV280 decrease down to 200 mAu on the downslope of the eluate peak.
Each daily eluted product pool was collected in a 10 L WAVE cell bag for viral inactivation. The eluate was adjusted to pH 3.5 ± 0.1 by addition of acid. The adjusted eluate was incubated with gentle rocking for 60 to 75 minutes and then readjusted to pH 5.0 by addition of 2 M Tris. After the viral inactivation step the material was 0.2 µm filtered using a ULTA Pure HC 0.6 / 0.2 µm 4" Capsule Filter and stored at 2-8°C until performance of the Capto S ImpAct step. No pressure increase was observed for either of the filtrations.
Table 3. Fibro PrismA chromatography method
| Description | Buffer | MV | Flow rate (L/h) |
| Equilibration | 20 mM NaP, pH 7.0 + 0.5 M NaCl | 5 | 39 |
| Load | Clarified harvest mAb8 | n/a | 39 |
| Wash 1 | 20 mM NaP, pH 7.0 + 0.5 M NaCl | 6 | 39 |
| Wash 2 | 50 mM Na-acetate, pH 5.5 | 6 | 39 |
| Elution | 50 mM Na-acetate, pH 3.5 | 6 | 39 |
| Re-eq | 20 mM NaP, pH 7.0 + 0.5 M NaCl | 1.5 | 39 |
| CIP | 0.5 M NaOH | 3 | 4 min |
| Re-eq | 20 mM NaP, pH 7.0 + 0.5 M NaCl | 8 | 39 |
| Storage* | 20% EtOH | n/a | 39 |
*Only last cycle of the day
Capto S ImpAct polishing step and Q membrane adsorber
The pooled and homogenized sub-batches from the ReadyToProcess MabSelect PrismA column was loaded onto a ReadyToProcess Capto S ImpAct 0.8 L column in two cycles. The pooled and homogenized sub-batches from the Fibro PrismA format was loaded onto the ReadyToProcess Capto S ImpAct 0.8 L column also in two cycles. In total, 4 cycles of the Capto S ImpAct step were performed. Column load in each cycle was between 74 to 76 g antibody/L resin corresponding to 70% of evaluated QB10. The chromatography method is shown in Table 4. The eluate peak was collected from a UV280 rise at 100 mAu to a decrease to 500 mAu on the downslope of the peak.
Some material from the capture step was left over and stored in cold room at 2-8°C for use in later studies. This was due to that more material than initially expected was produced in the cultivation and there are limitations in the loading capacity of the Capto S ImpAct column.
Table 4. Capto S ImpAct chromatography method
| Description | Buffer | CV | Flow rate (residence time) |
| Equilibration | 50 mM Sodium Acetate, 50mM NaCl pH 5.0 | 5 | 5.4 min |
| Load | Adjusted VI pool, pH 5.0 ± 0.2 | n/a | 5.4 min |
| Wash | 50 mM Sodium Acetate, 50mM NaCl pH 5.0 | 5 | 5.4 min |
| Elution | 50 mM Sodium Acetate, 200 mM NaCl pH 5.0 | 7 | 5.4 min |
| Strip | 1 M NaCl | 3 | 5.4 min |
| CIP | 0.5 M NaOH | 3 | 5.4 min |
| Re-eq | 50 mM Sodium Acetate, 50mM NaCl pH 5.0 | 5 | 5.4 min |
| Storage* | 20% EtOH | n/a | 5.4 min |
*Only last cycle of the day
Following elution from the cation exchanger each separate product pool was sampled and then instantly diluted and pH adjusted to the loading condition (pH 6.0, conductivity 10 mS/cm) of the Q Membrane Adsorber by addition (ratio 4:1) of conditioning buffer. The pH and conductivity of the adjusted Capto S Impact eluate was controlled and then loaded directly onto the ReadyToProcess Q membrane adsorber.
Each Capto S ImpAct sub-batch was loaded separately after adjustment, onto the Q step giving in total 4 cycles of the anion exchanger. Membrane load in each cycle was between 127 to 137 g antibody/L membrane. The chromatography method is shown in Table 5. The flow through peak was collected from a UV280 rise at 100 mAu to a decrease to 100 mAu on the downslope of the peak.
Table 5. Adsorber Q chromatography method pH 6.0
| Description | Buffer | MV | Flow rate (L/h) |
| Equilibration | 50 mM Sodium Acetate, pH 6.0 | 20 | 120 |
| Load | Adjusted Capto S ImpAct eluate | n/a | 120 |
| Wash | 50 mM Sodium Acetate, pH 6.0 | 10 | 120 |
| Strip | 1 M NaCl | 5 | 120 |
| CIP | 1 M NaOH | 5 + 30 min hold | 120 |
| Re-eq | 50 mM Sodium Acetate, pH 6.0 | 20 | 120 |
| Storage* | 20% EtOH | n/a | n/a |
*Only last cycle of the day
UF/DF and final filtration
The final purified material from the Q membrane adsorber step was concentrated and buffer exchanged to the formulation buffer. The two sub-batches from the ReadyToProcess MabSelect PrismA column and Fibro PrismA process trains were pooled prior to transfer into the product bag connected to the ÄKTA readyflux. The two materials originating from the different capture formats were processed separately in the ultrafiltration/diafiltration (UF/DF) step generating two final products.
A UFP-30-C-9A hollow fiber filter 30 kDa, 1.15 m2 was used for the concentration and diafiltration process, one new filter for each batch.
The UF/DF process was run automatically by UNICORN™ control software and included a normal water permeability (NWP) test, filter equilibration with buffer, product transfer into the system product bag, initial concentration to 5 L followed by 6 volumes of diafiltration using the formulation buffer. After the buffer exchange the product was emptied from the system into 5 L bags. The filters and system were flushed with 1 L of formulation buffer and thereafter emptied in to the same 5 L bag to maximize recovery. Process data can be seen in Table 6. The feed flow was adjusted slightly for the second batch since the TMP was considered a bit high in the first batch.
The final retentate was filtered into storage bags using a pump and an ULTA Pure HC 0.6/0.2 µm Capsule Filter. The final material was stored at 2-8°C.
Table 6. UF/DF process running parameters
| Sub-batch | Filter load (g/m2) | Shear rate (1/s) | Feed flow (L/min) | TMP (bar) |
| Batch 1 | 90 | 8000 | 10.8 | 1.7 ± 0.1 |
| Batch 1 | 90 | 8000 | 10.8 | 1.7 ± 0.1 |
| Batch 2 | 91 | 7400 | 10.0 | 1.2-1.3 |
Analytical methods
Cedex™ Bio was used for in-process concentration determination of mAb8 in cell supernatant to be able to adjust and adapt volume to load on the capture step. A Biacore™ system was used for concentration determination of mAb8 in cellsupernatant and all other process intermediates for recovery calculations. A280 was used for in-process concentration determination of mAb8 in the polishing steps to calculate
HCP and residual Protein A was analyzed using Gyrolab technology. DNA was analyzed using qPCR. Bioburden was analyzed by calculating colony forming units on agar plates after membrane filtration and incubation. Endotoxin levels were determined by measuring level of p-nitroaniline (at 405 nm).
| Description | Product code |
| XDX storage bag 200 L, 3 port | 888-1259-F |
| XDM-200 PLUS Bag | 888-0155-C |
| ÄKTA readyflow kit with ReadyMate | 29007855 |
| ÄKTA readyflux flow kit plus | 29187382 |
| ULTA Pure HC 0.6 / 0.2 µm 2" Capsule Filter, 6" of tubing | 12410093 |
| ULTA Pure HC 0.6 / 0.2 µm 4" Capsule Filter, 6" of tubing | 12410094 |
| ReadyCircuit RMRM Jumper 5 ft | 12410116 |
| ReadyCircuit Jumper T Manifold, 6 ports, 6" of 0.5" tubing | 12410183 |
| ReadyCircuit Jumper Y Manifold, 3 ports, 6" of 0.5" tubing | 12410191 |
| Cellbag bioreactor 2 L | CB0002L11-31 |
| Cellbag bioreactor 10 L | CB0010L11-31 |
| Sensor Assembly with a Scilog pressure sensor and 6 in (152 mm) of AdvantaPure™ Reinforced Silicone 0.375 in (10 mm) ID tubing terminating with ReadyMate connectors. | 28979471 |
| ReadyCircuit 20 L Hanging/Pillow Bag, needleless CLAVE sample port, 4 ports, 1 ft of C-Flex® 374 0.5" | 12410225 |
| ReadyCircuit 50 L Pillow Bag, with a needleless CLAVE sample port, 4 ports, 1 ft of C-Flex 374 0.75" | 12410229 |
| Fibro PrismA 160 mL prototype | n/a |
| ReadyToProcess PrismA column 0.8 L (80/150) | 29420989 |
| ReadyToProcess Capto S ImpAct 0.8 L (80/150) | 29287577 |
| ReadyToProcess Adsorber Q 400 4 mm | 17372109 |
| ReadyCircuit PSIL jumper | 28979432 |
| ReadyCircuit 5 L Hanging/Pillow Bag, needleless CLAVE sample port, 3 ports, 1 ft of C-Flex® 374 0.375" | 12410220 |
| ReadyCircuit 10 L Hanging/Pillow Bag, needleless CLAVE sample port, 3 ports, 1 ft of C-Flex®374 0.5" | 12410222 |
| ReadyMate connectors mini TC 50 pieces | 28936707 |
| ReadyMate connectors TC 50 pieces | 28956890 |
| UFP-30-C-9A hollow fiber filter 30 kDa, 1.15 m2 | RTPUFP-30-C-9S |
| 50 mm TC clamp | 56410668 |
| 25 mm TC clamp | 56410666 |
| XDX storage bag 500 L, 3 port | 888-1260-F |
| Syringes, 20 mL and 50 mL | N/A |
| Description | Product code |
| XDX storage bag 200 L, 3 port | 888-1259-F |
| XDM-200 PLUS Bag | 888-0155-C |
| ÄKTA readyflow kit with ReadyMate | 29007855 |
| ÄKTA readyflux flow kit plus | 29187382 |
| ULTA Pure HC 0.6 / 0.2 µm 2" Capsule Filter, 6" of tubing | 12410093 |
| ULTA Pure HC 0.6 / 0.2 µm 4" Capsule Filter, 6" of tubing | 12410094 |
| ReadyCircuit RMRM Jumper 5 ft | 12410116 |
| ReadyCircuit Jumper T Manifold, 6 ports, 6" of 0.5" tubing | 12410183 |
| ReadyCircuit Jumper Y Manifold, 3 ports, 6" of 0.5" tubing | 12410191 |
| Cellbag bioreactor 2 L | CB0002L11-31 |
| Cellbag bioreactor 10 L | CB0010L11-31 |
| Sensor Assembly with a Scilog pressure sensor and 6 in (152 mm) of AdvantaPure™ Reinforced Silicone 0.375 in (10 mm) ID tubing terminating with ReadyMate connectors. | 28979471 |
| ReadyCircuit 20 L Hanging/Pillow Bag, needleless CLAVE sample port, 4 ports, 1 ft of C-Flex® 374 0.5" | 12410225 |
| ReadyCircuit 50 L Pillow Bag, with a needleless CLAVE sample port, 4 ports, 1 ft of C-Flex 374 0.75" | 12410229 |
| Fibro PrismA 160 mL prototype | n/a |
| ReadyToProcess PrismA column 0.8 L (80/150) | 29420989 |
| ReadyToProcess Capto S ImpAct 0.8 L (80/150) | 29287577 |
| ReadyToProcess Adsorber Q 400 4 mm | 17372109 |
| ReadyCircuit PSIL jumper | 28979432 |
| ReadyCircuit 5 L Hanging/Pillow Bag, needleless CLAVE sample port, 3 ports, 1 ft of C-Flex® 374 0.375" | 12410220 |
| ReadyCircuit 10 L Hanging/Pillow Bag, needleless CLAVE sample port, 3 ports, 1 ft of C-Flex®374 0.5" | 12410222 |
| ReadyMate connectors mini TC 50 pieces | 28936707 |
| ReadyMate connectors TC 50 pieces | 28956890 |
| UFP-30-C-9A hollow fiber filter 30 kDa, 1.15 m2 | RTPUFP-30-C-9S |
| 50 mm TC clamp | 56410668 |
| 25 mm TC clamp | 56410666 |
| XDX storage bag 500 L, 3 port | 888-1260-F |
| Syringes, 20 mL and 50 mL | N/A |