The next industrial revolution is digital
Since the dawn of manufacturing, we have seen several industrial revolutions, all enabled by significant leaps in productivity. Each revolution marked a major shift in the economy and changed the way companies do business to meet customer demands. The last big revolution came with the introduction of computers on manufacturing lines. This gave way to the advent of automation, which increases efficiency and speed. It also minimizes process variability caused by humans. Although slower to adopt automation than other industries, the pharmaceutical industry continues to find ways to use it for improving quality and speed to market. Now, another transformation is taking place that will pave the way for even more changes to how the industry approaches drug manufacturing.
In the latest phase, often referred to as Industry 4.0 (Fig 1), cyber-physical systems control and monitor activity through computer-based algorithms. Although this might seem like science fiction for an industry that is arguably still working through Industry 3.0, the benefits of embracing digital transformation are too substantial to ignore. Experts expect Industry 4.0 to trigger a quantum leap in productivity, because these connected machines and software systems can enhance the human workforce in new and exciting ways. In bioprocessing, many possibilities could be realized over time through this revolution. For example, in the near future, tools of Industry 4.0 could minimize maintenance downtime and spare part inventory for process equipment. These tools could also enable continuous improvement of process robustness and efficiency.
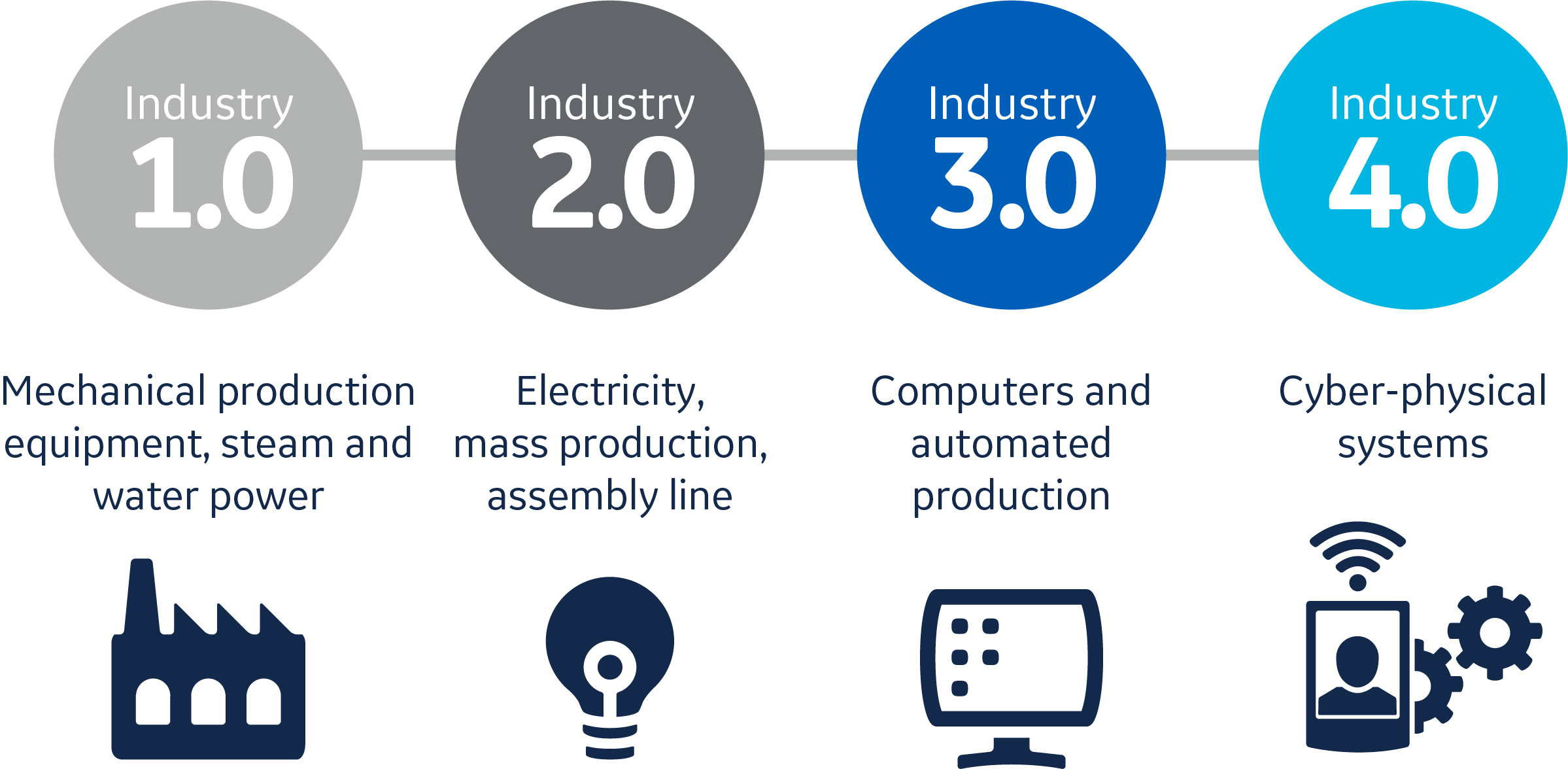
Fig 1. The impact of cloud computing, machines connected to the internet, and more powerful software is expected to lead to a quantum leap in bioprocess productivity.
An evolving biopharma industry needs connected data
The landscape of today’s pharmaceutical industry looks much different than it did 20 years ago. An impressive growth in biopharmaceutical innovation has brought new possibilities in patient care and challenges in manufacturing. Many new and innovative approaches to medicine involve niche drugs that target smaller patient populations. This means drugs are manufactured in much lower volumes. As a result, manufacturers must improve their operational efficiency to reduce overall drug development and manufacturing costs, so they can meet the public’s demand for access to new drugs and reasonable pricing. The Industry 4.0 paradigm will help achieve this goal.
According to the Industry 4.0 paradigm, an isolated, vendor-centric world is inefficient. This is true because end users of manufacturing software systems often must develop costly, proprietary point solutions. This approach limits the ability to leverage the best expertise, because it inhibits the transfer of already-developed solutions, collaboration, and communication across multiple companies. In a new world of manufacturing, open systems that leverage standards for interoperability and data exchange, such as open platform communications (OPC) and other applicable standards yet to be developed, will liberate data. Data can then be connected and contextualized for use in a large variety of applications. The goal is to facilitate better decision-making, identify improvement opportunities, and, ultimately, optimize operations. Some areas where connected data in bioprocessing could offer productivity gains include:
- Uptime — When equipment is connected, visibility into its condition improves. Instead of waiting for something to break, a manufacturer can monitor the condition of its equipment and become predictive about maintenance. From a supply chain perspective, connected data also allows visibility into which materials are needed at what time. This insight can reduce both unnecessary inventory and operating delays.
- Process understanding — The operations systems used today, such as control automation systems, manufacturing execution systems (MES), and laboratory information management systems (LIMS), are designed to make sure a batch runs properly and smoothly every time. However, the data these systems generate is typically not aggregated across batches or connected between systems in a way that enables better decision-making. To achieve a more holistic view of the plant and process performance, a manufacturer needs to unlock the data from these silos. This step will shorten the time for carrying out investigations and other process troubleshooting.
- Robustness and productivity — As data becomes more readily available, it is possible to proactively improve understanding of the sources of variability in manufacturing processes. This proactive approach will reduce the number of investigations needed and possibly even eliminate batch failures. Also, data access will allow a manufacturer to identify process improvement opportunities and implement changes to increase productivity. It is common to find that output from a biomanufacturing process can be significantly improved by optimizing control parameters within ranges already filed with and approved by regulatory authorities.
- Compliance — When connectedness between systems and data is leveraged to enable new applications, data integrity becomes even more important. As this happens, new opportunities to maintain compliance will likely be uncovered.
Digital transformation of biomanufacturing will be driven by observing the performance of processes and workflows to determine where improvements can be made. This requires a connected infrastructure where the data can flow and be visualized. It also requires that teams connect, so people can collaborate around desired outcomes, as discussed in a BPOG paper (1). From there, the data to do predictive analytics can be applied, such as building knowledge and focusing on specific, and increasingly bigger, problems. As this connection scales up successfully, fully integrated prediction models can be achieved, leading to overall process optimization.
How to succeed with digital transformation
Consider these two aspects:
1. Implementing new technology
Industry 4.0 is about building tools that leverage and interpret data from various systems, so systems can operate and communicate with each other (Fig 2). Data is the raw material that drives continuous improvement. If data cannot be accessed across a facility, the knowledge needed to make meaningful and effective changes will not be available. If data is accessible, a manufacturer can discover inefficiencies in its processes. Although a few major improvement opportunities might be identified, the quantum leap in efficiency will come from many small, incremental gains that, together, result in significant cost savings. Any big or small improvements made over time and introduced systematically will multiply, rather than add up, across manufacturing operations.
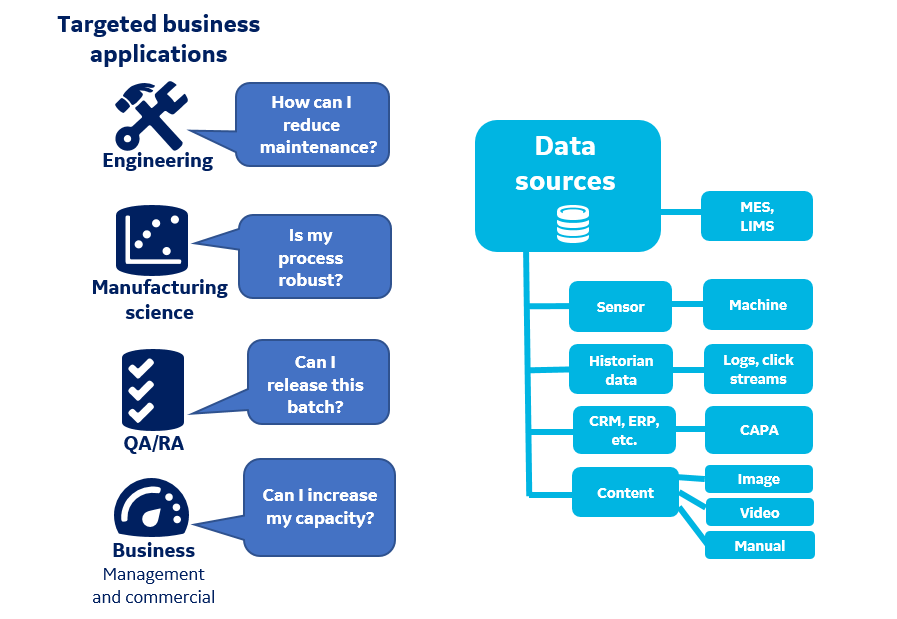
Fig 2. Industry 4.0 requires unlocked and available data. Dramatic productivity increases will come from introducing many small gains across the enterprise. In bioprocessing, improvements can be made across traditionally isolated areas.
2. Effective change management within an organization
A change in culture usually comes from the top down, so embracing and executing something that could cause fear and resistance (especially due to job security concerns) should begin with a company’s senior leadership. The environment must encourage and embrace the change. Succeeding with digital transformation also requires a review of how external relationships function. For example, the vendor/manufacturer relationship in biomanufacturing is typically very transactional. However, following the discussed principles of open interoperable systems and collaboration will benefit both suppliers and end users through shared data and knowledge.
Case study – Cytiva and Biogen collaboration
A case study by Cytiva and Biogen shows how collaboration and data sharing can be mutually beneficial (2). For the study, the question was: How is process variability managed in today’s industry? Specifically, the industry has insufficient understanding of the impact of raw material variability and how to respond to it. Even with rigorous process development, key uncertainties about process robustness still exist in the commercial phase of manufacturing. The related risks are typically addressed in a reactive manner. This approach leads to lengthy investigations, heroic efforts to rectify process performance, and even batch rejections. Therefore, there is an opportunity to become proactive with this issue, in order to continuously:
- assess variability and retire risks
- build shared process knowledge
- improve efficiency
This case study was performed on a legacy process that had been replaced. It was carried out by retrospectively analyzing seven years of data. The objective was to mimic the knowledge and, even more importantly, identify gaps in that knowledge at every point in time. The study’s approach was to combine process data from the manufacturer with detailed raw material data from a supplier, in order to create a holistic view of process performance.
Cytiva and Biogen were able to proactively identify and detect potential variability risks that were missed when the process was run. They also successfully predicted a critical quality attribute. That prediction model can then detect other potential variability risks which, in turn, helps identify mitigation activities. Using this collaborative approach, manufacturing processes become connected, creating a need for long-term strategic partnerships around process life cycles. This is a major change of mindset from today’s typical supplier/end user relationship, because this level of openness requires a lot of trust between the parties.
Supplier data can help users to make smart choices from the beginning and better understand the risks associated with variability. Such collaboration can also aid in creating control strategies with a high chance of success. Later, when trying to understand the variability, end users can determine how much effort to put into their characterization relative to risk reduction. Suppliers can provide knowledge about how materials perform in the targeted applications and assist with making those risk assessments. Depending on what is learned during process development, suppliers can also help to evaluate possible control strategy options. The relationship then becomes a true collaboration where each member provides guidance and support and works toward the same goal.
What biomanufacturers can do to prepare for Industry 4.0
Overall, to realize the long-term benefits of digital transformation, start working on a strategy now. Develop a vision and complement that by pinpointing any problems that realistically could be solved by deeper data insights. Leverage short-term successes, using the returns from these to fund the next project. This way, it is possible to implement programs that focus on business outcomes rather than technology, which facilitates the human aspects of digital transformation. Finally, keep in mind that digital transformation requires collaboration between manufacturers, suppliers, and other business partners. Identify those who are willing to share this journey through a foundation of trust, dedicated collaboration, and open communication.
References
1. BioPhorum Operations Group (BPOG). The development of a Digital Plant Maturity Model to aid transformation in biopharmaceutical manufacturing [Online] https://www.biophorum.com/digital-plant-maturity-model-paper/ (2016).
2. Malmquist G, Jiang C; Smart: a synergistic life cycle approach to understand and control raw material variability through collaborative process analytics; Recovery of Biological Products RXVII, Bermuda, June 2016.
Read about digital transformation at one of Cytiva's cell culture media production facilities.