This article describes how to ensure a scalable process for buffer management from process development to manufacturing using ÄKTA avantTM chromatography system and BioProcessTM IC System for inline conditioning (IC). It also explains how ÄKTA avantTM can be used to obtain molar recipe information instead of doing tedious and time-consuming titration in the laboratory.
Buffer control has an important role in biomanufacturing, because it is critical for overall process control. Therefore, a large part of process development (PD) consists of identifying the right buffers to ensure the optimal outcome from the capture, intermediate, and polishing steps of protein purification.
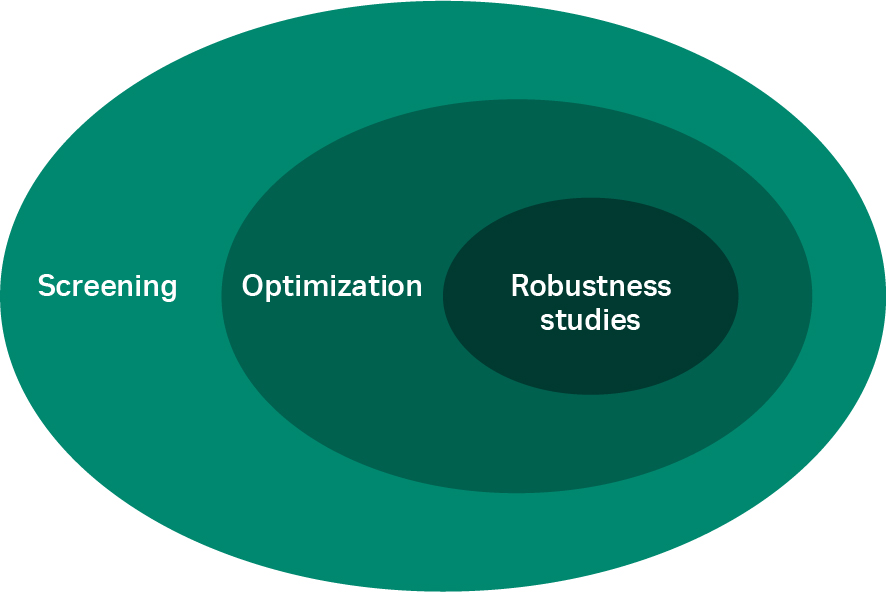
Through screening, a large area is explored in the space of the potential buffer conditions to identify the areas of interest for additional, more quantitative studies (i.e., optimization). After the optimal buffer condition settings have been determined, the robustness of the process is verified with limited variations in the buffer conditions (Fig 1).
Fig 1. The process development space from screening to robustness studies used to identify the operating buffer conditions.
Traditional buffer preparation – a bottleneck in process development and manufacturing
During process development, the traditional way of preparing buffers is by weighing salts, stirring in water, and then filling up to the line, while titrating with a strong acid or base. This method constitutes a bottleneck, because the process is time consuming and there are many buffers to prepare.
When going into production, the optimal buffer conditions have been obtained and the number of required buffers is greatly reduced. However, traditional buffer preparation methods can lead to other, even more serious issues. This is mainly due to the high buffer volumes that are required in manufacturing. These challenges include:
- the heavy infrastructure needed to support tanks that might contain tens of thousands of liters of buffers.
- the labor and time needed to clean and validate those tanks.
- the labor and time needed to formulate, document, and qualify the buffers.
- transportation of buffers, for example, from a storage site to the place of use.
Therefore, it is desirable to have a leaner, more efficient approach that allows a higher level of automation, standardization of storage materials, and elimination of unnecessary storage volumes.
Buffer management during process development
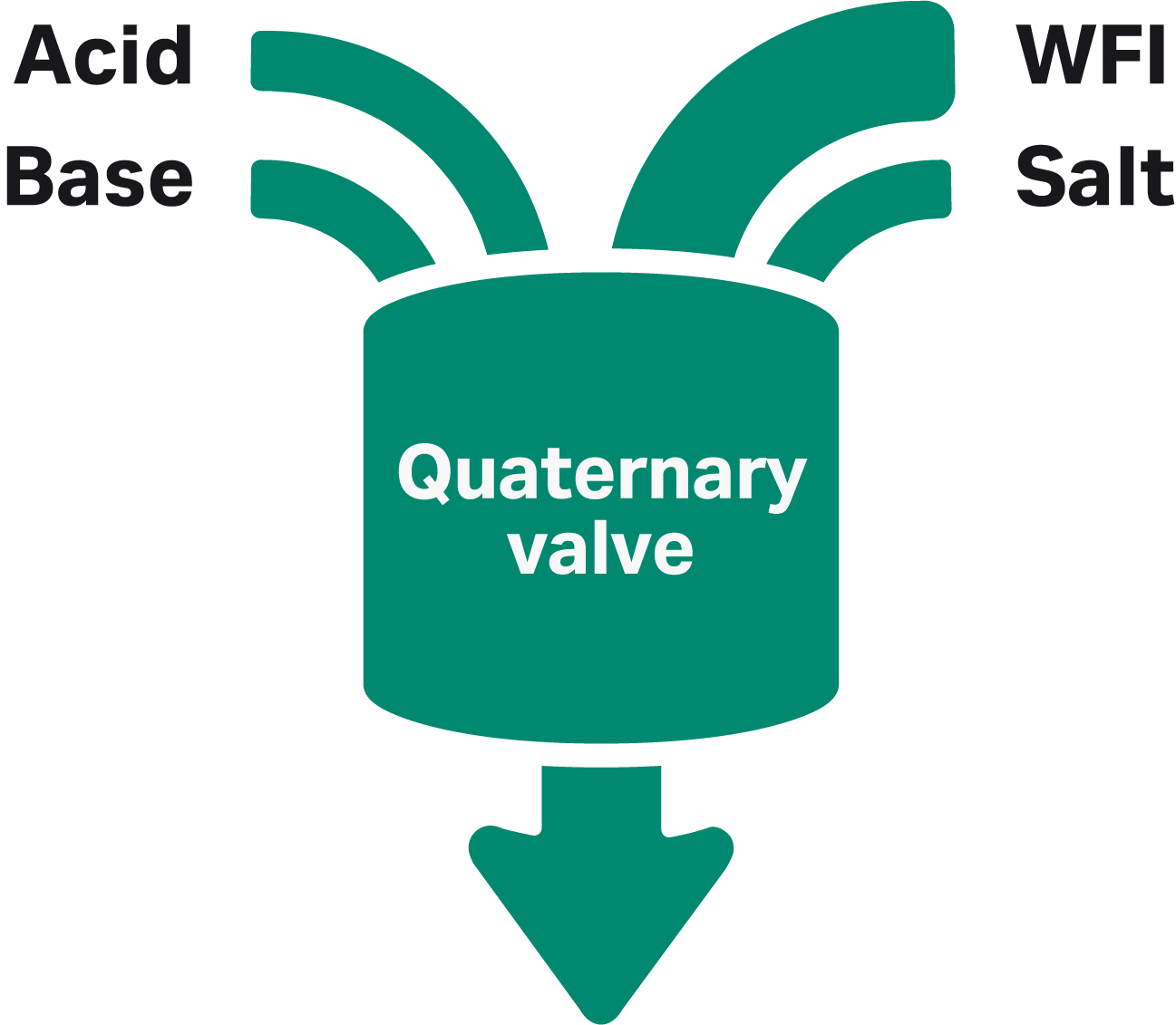
To reduce the buffer preparation bottleneck in process development, the ÄKTA avantTM system can be used to produce buffers at the point of use from a few standardized stock solutions. The buffers are formulated by mixing appropriate amounts of the stock solutions of acid, base, salt, and water using the four inlets of the quaternary valve (Fig 2).
Fig 2. ÄKTA avantTM quaternary valve. WFI is water for injection.
The following features included in ÄKTA avantTM support for buffer preparation:
- ÄKTA avantTM can be used to produce multiwell plates containing buffers, for use with PreDictorTM 96-well plates in high-throughput process development (HTPD). These plates are prefilled with BioProcess chromatography resins (1).
- Buffers can be prepared in the ÄKTA avantTM system using the BufferPro tool (2), where the input is the desired pH, buffer, and salt concentration. BufferPro then controls the quaternary valve and automatically adjusts the appropriate proportions of acid, base, salt, and water. BufferPro contains 19 different buffer systems to choose from. This easy-to-use tool is integrated in the predefined phases of the method editor.
- If process development requires a buffer system not available in BufferPro, buffer mixing can be programmed using the quaternary valve instruction in the method editor. The input is the desired partial proportions of acid, base, salt, and water. Use of the quaternary valve instruction gives more freedom to choose different buffer settings but requires more knowledge about the buffer composition.
Buffer management at production scale
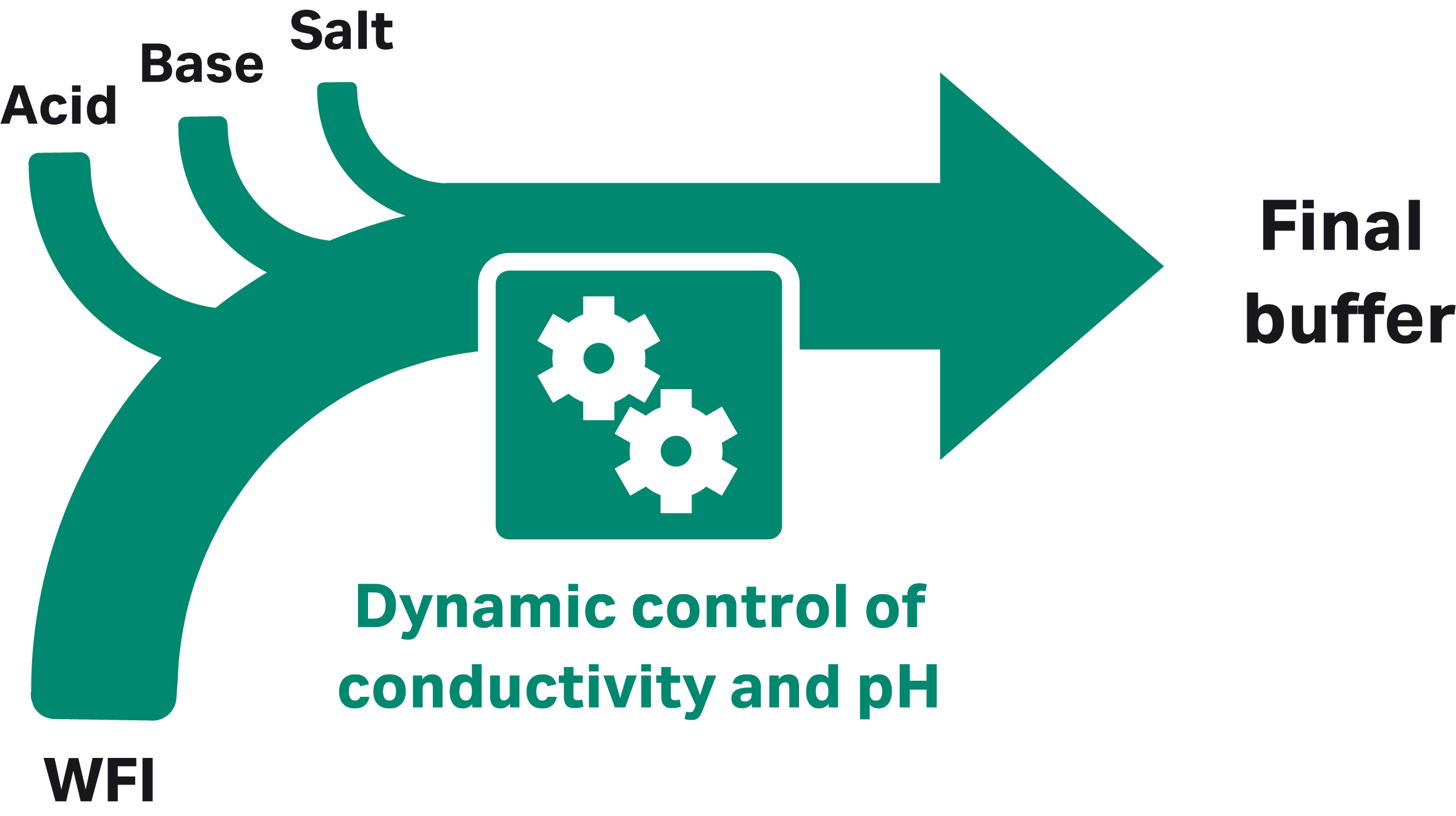
At production scale with at least four pumps, the BioProcessTM IC System can prepare the required buffers from concentrated stock solutions automatically, reducing the need for manual intervention. Buffers of the desired concentration and pH are prepared from concentrated stocks of acid, base, a salt or additive, plus water for injection (WFI; Fig 3). The use of concentrated stock solutions substantially reduces both floor space requirements and tank volumes.
Fig 3. IC approach with four pumps, each delivering a different component.
This approach of making buffers at the point of use from a few standardized solutions can be called “inline conditioning.” This is true because the conditions, which are pH, conductivity, salt, or buffer concentration, are achieved inline at the point of mixture.
In addition, many different buffers can be prepared from the same set of concentrates, which further streamlines buffer preparation. For improved accuracy in formulation and consistency between preparations, different feedback modes featuring the built-in dynamic control functionality can be selected.
Scaling up and scaling down – molar recipes make it simple
The molar recipe is the molar concentration of each of the components (acid and base) in a buffer of a given concentration and at a given pH. The concept of the buffer molar recipe is central for IC. Molar recipes are needed either to run the BioProcessTM IC system in flow feedback mode or in the initial stages of pH or conductivity feedback mode to ensure quick convergence to the target values.
Scaling the buffer formulation up and down from single components is simple, because the molar composition or “molar recipe” stays the same. The proportions going into the different quaternary inlets in ÄKTA avantTM or coming from the different pumps in IC can be easily calculated from the molar recipe and stock solution concentration.
Because the molar recipe is the same in both scales, it can be obtained using the ÄKTA avantTM and used at production scale with an BioProcessTM IC System (scaling up). Another option is to obtain the molar recipe from the large-scale process and use it as a starting point for robustness studies in the ÄKTA avantTM (scaling down).
Some molar recipes can be obtained with the help of commercial software, for example using the “Explore proportions” calculator of the BufferPro tool in UNICORNTM 6 and 7. However, many recipes are impossible to obtain with software due to complexity in their composition. In these cases, the only way to obtain an appropriate molar recipe is through experiments in the laboratory. The molar recipe can also be obtained by running the BioProcessTM IC System in pH feedback mode, but it may require large volumes of stock solutions.
How to obtain a molar recipe for a given pH using scouting
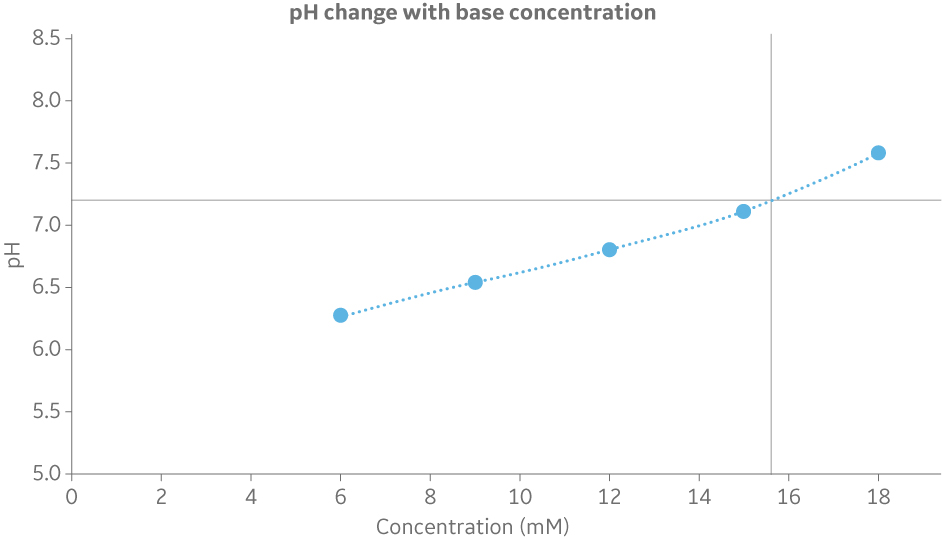
Whereas pH feedback is available in the BioProcessTM IC System, scouting can be used in the ÄKTA avantTM to explore how the pH varies as a function of different relative proportions between the acid and the base. The scouting function makes it possible to do multiple runs in an automated way to build a pH titration curve (Fig 4). The titration curve is then used to obtain the molar recipe.
Fig 4. Titration curve to determine the base concentration at a specified pH. In this example 20 mM phosphate with 200 mM NaCl at pH 7.2 gives 15.6 mM of the base component (Na2HPO4).
The percentage of acid and base are based on the concentrations of the stock solutions and final buffer. To build the pH titration curve, different combinations of acid and base percentages are chosen for the runs. The only detail to keep in mind is to constrain these percentages to each other in such a way that the buffer concentration is maintained. The fraction collector can be used to collect each buffer for offline measurements if desired.
The molar recipe is obtained from the pH titration curve constructed in Microsoft Excel®. After the graph is produced, the base concentration for the target pH can be identified. From the base concentration, it is then possible to calculate the acid concentration given the final buffer concentration.
The example in Figure 5 shows a comparison of molar recipe determination using ÄKTA avantTM and IC system.
(A)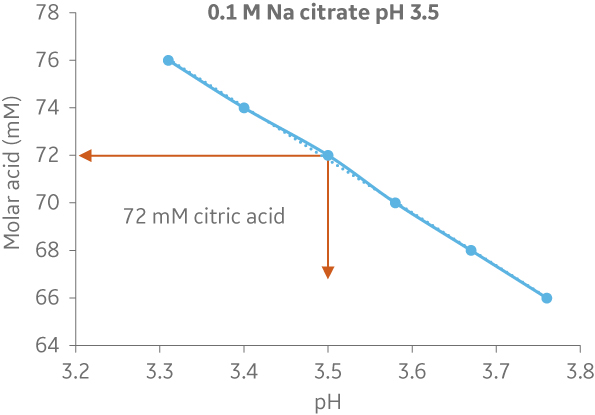
|
(B)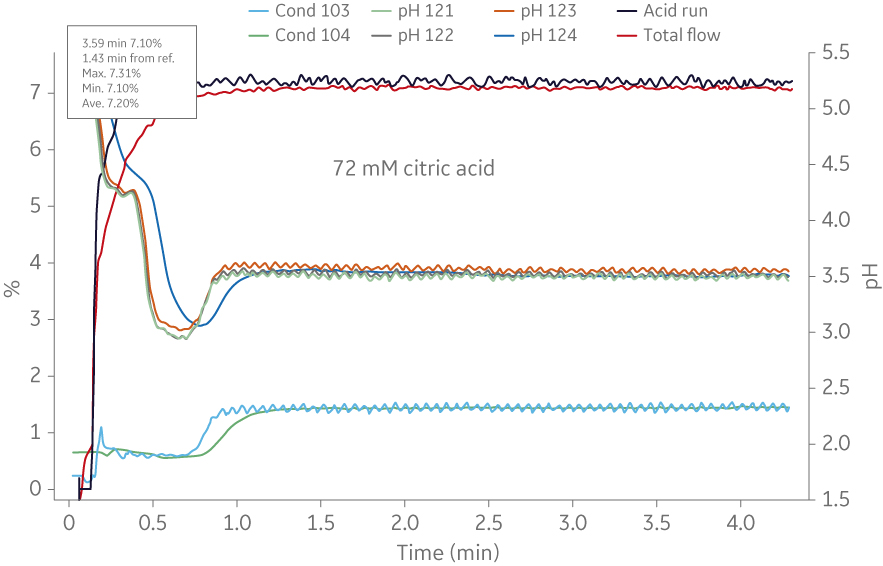
|
Fig 5. Comparison of molar recipe determination of a buffer (72 mM citric acid/ 28 mM trisodium citrate) using ÄKTA avantTM and IC system. (A) Shows the titration curve obtained from scouting runs on ÄKTA avantTM. In this case the molar recipe could be obtained using 500 ml buffer, and (B) shows an example from the IC system where 50L of buffer was needed to obtain the same molar recipe. The buffer consumption was increased 100-fold when using the BioProcessTM IC System.
The fraction collector supports offline measurements of buffers formulated inline, and the use of scouting for molar recipe determination can be automated. Both ÄKTA avantTM and IC can be run with UNICORNTM software allowing easy conversion of methods between systems. All these features facilitate scaling up and scaling down of the inline buffer preparation procedure.
Buffer preparation comparison
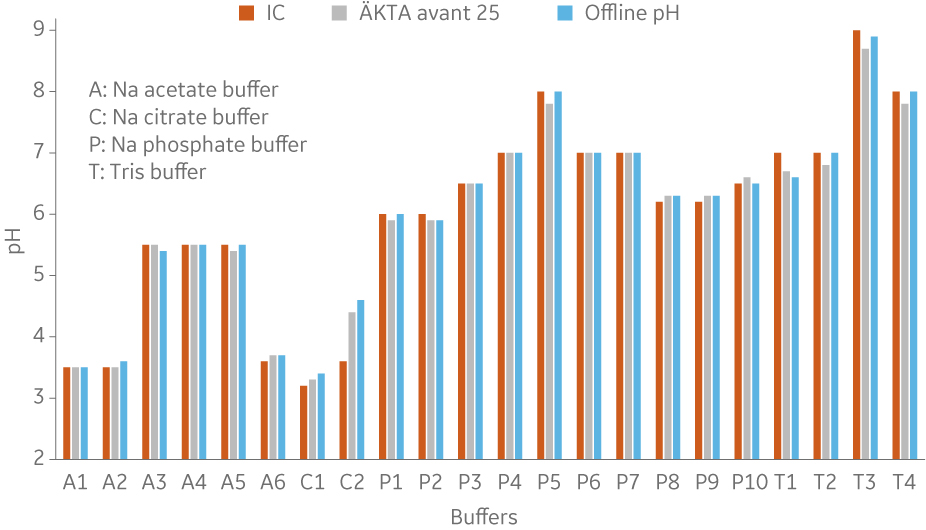
To show comparability between buffers formulated in BioProcessTM IC System and ÄKTA avantTM, buffers from four common buffer types were prepared using both systems and the same stock solutions and recipes (Fig 6 to 10).
Fig 6. pH of different buffers using built-in pH probes in both systems. Comparison with offline pH measurement of the buffers produced in ÄKTA avantTM using a standalone pH meter.
(A)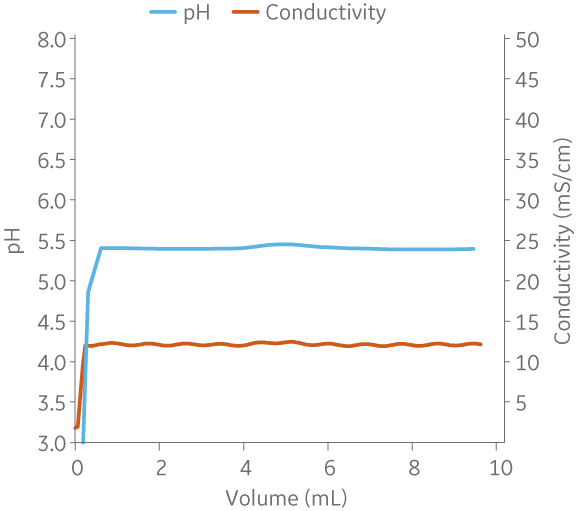
|
(B)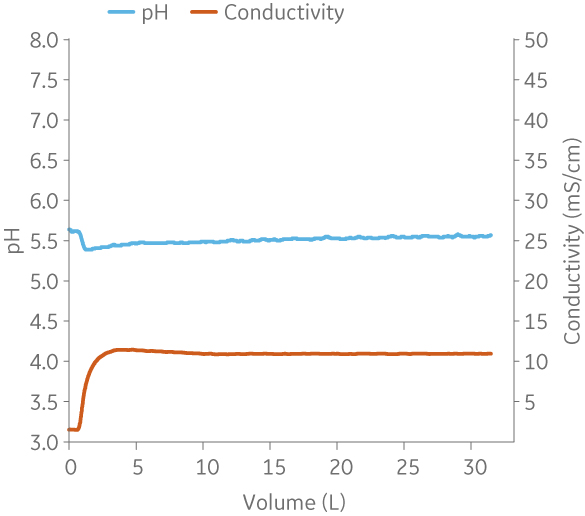
|
Fig 7. Example of a UNICORNTM run. 25 mM Na acetate buffer, 100 mM NaCl at pH 5.5 from (A) ÄKTA avantTM 25 and (B) the BioProcessTM IC System.
(A)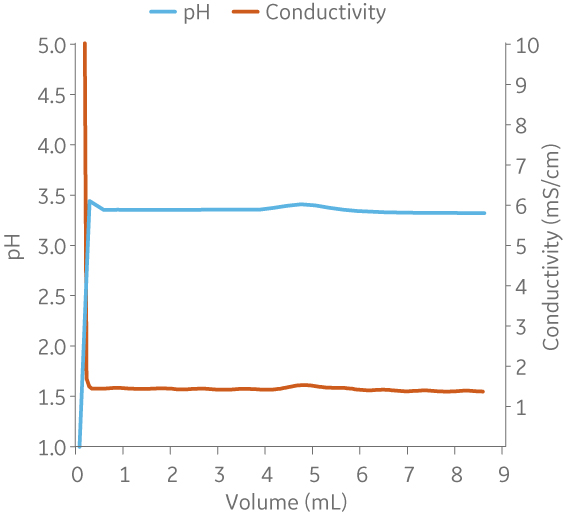
|
(B)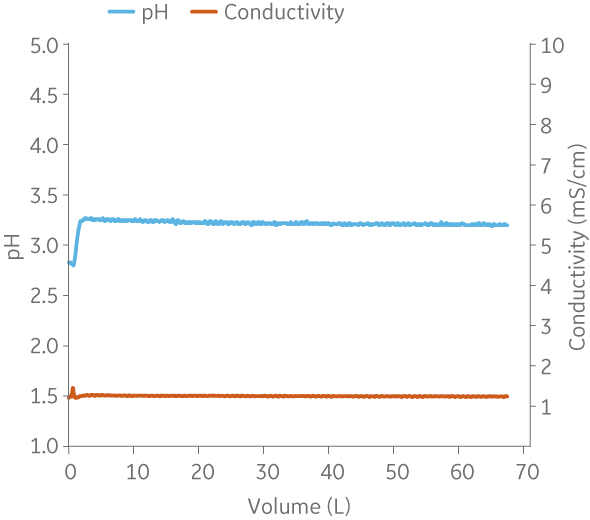
|
Fig 8. Example of a UNICORNTM run. 25 mM Na citrate buffer at pH 3.2 from (A) ÄKTA avantTM 25 and (B) the BioProcessTM IC System.
(A)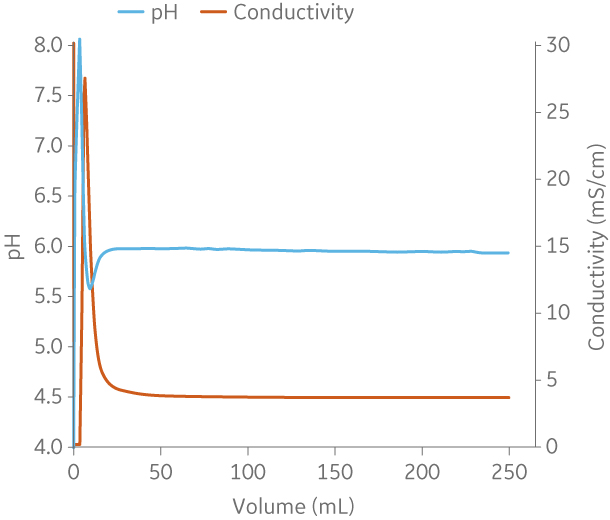
|
(B)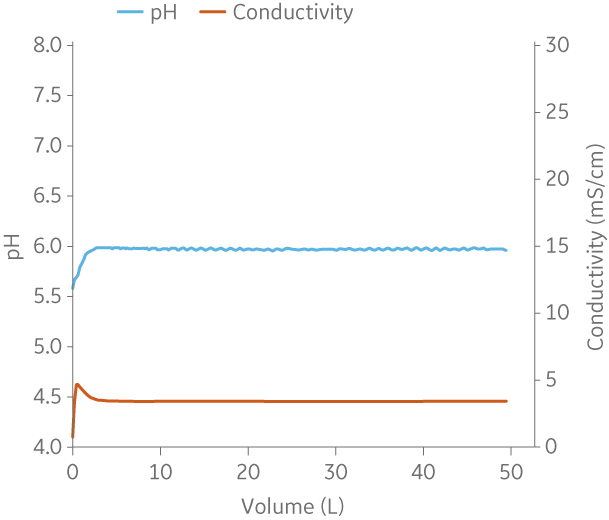
|
Fig 9. Example of a UNICORNTM run. 50 mM Na phosphate buffer at pH 6.0 from (A) ÄKTA avantTM 25 and (B) the BioProcessTM IC System.
(A)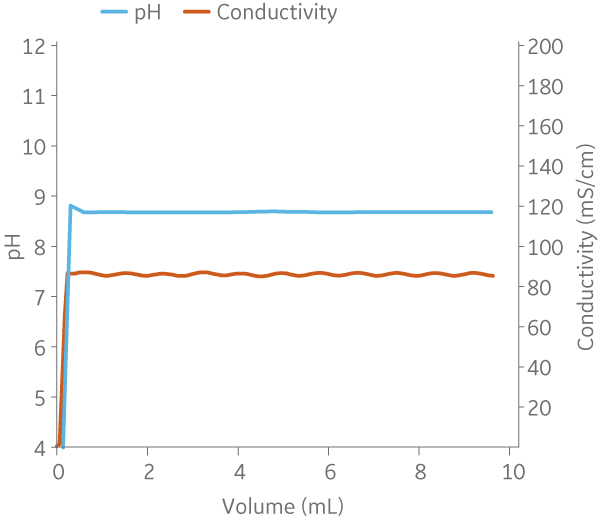
|
(B)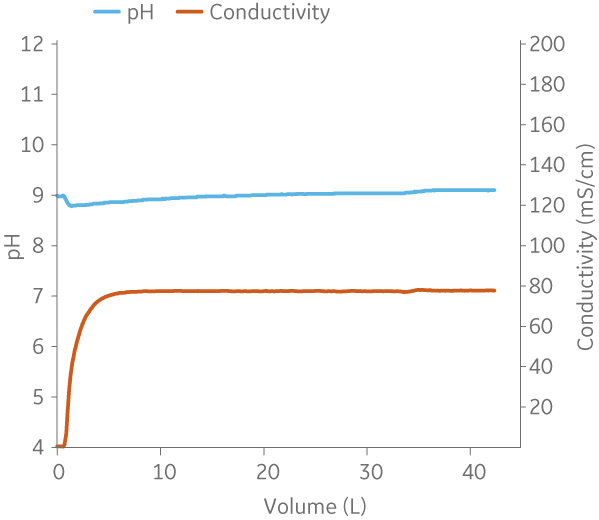
|
Fig 10. Example of a UNICORNTM run. 25 mM Tris 1 M NaCl buffer at pH 9.0 from (A) ÄKTA avantTM 25 and (B) the BioProcessTM IC System.
Summary – systems to automate and streamline buffer preparation from process development to production
Producing buffers for large-scale chromatography requires significant resources. These resources include time, money, and space to store the buffers. Inline conditioning, where buffers are formulated from single-component stock solutions, is one way to address these challenges, saving both floor space and tank volumes. Buffer testing of the formulation strategy (i.e., determination of the molar recipe) can be done in small scale using the ÄKTA avantTM and verified at large scale with the BioProcessTM IC System.
The ÄKTA avantTM system helps to reduce the buffer preparation bottleneck through screening, optimization, and robustness studies during process development. This article shows that the ÄKTA avantTM can also be used to support IC implementation.
(A)
|
(B)
|
Fig 11. (A) ÄKTA avantTM system and (B) BioProcessTM IC System.
Watch our webinar: From ÄKTA avantTM chromatography system to inline conditioning
Learn more about large-scale buffer management.