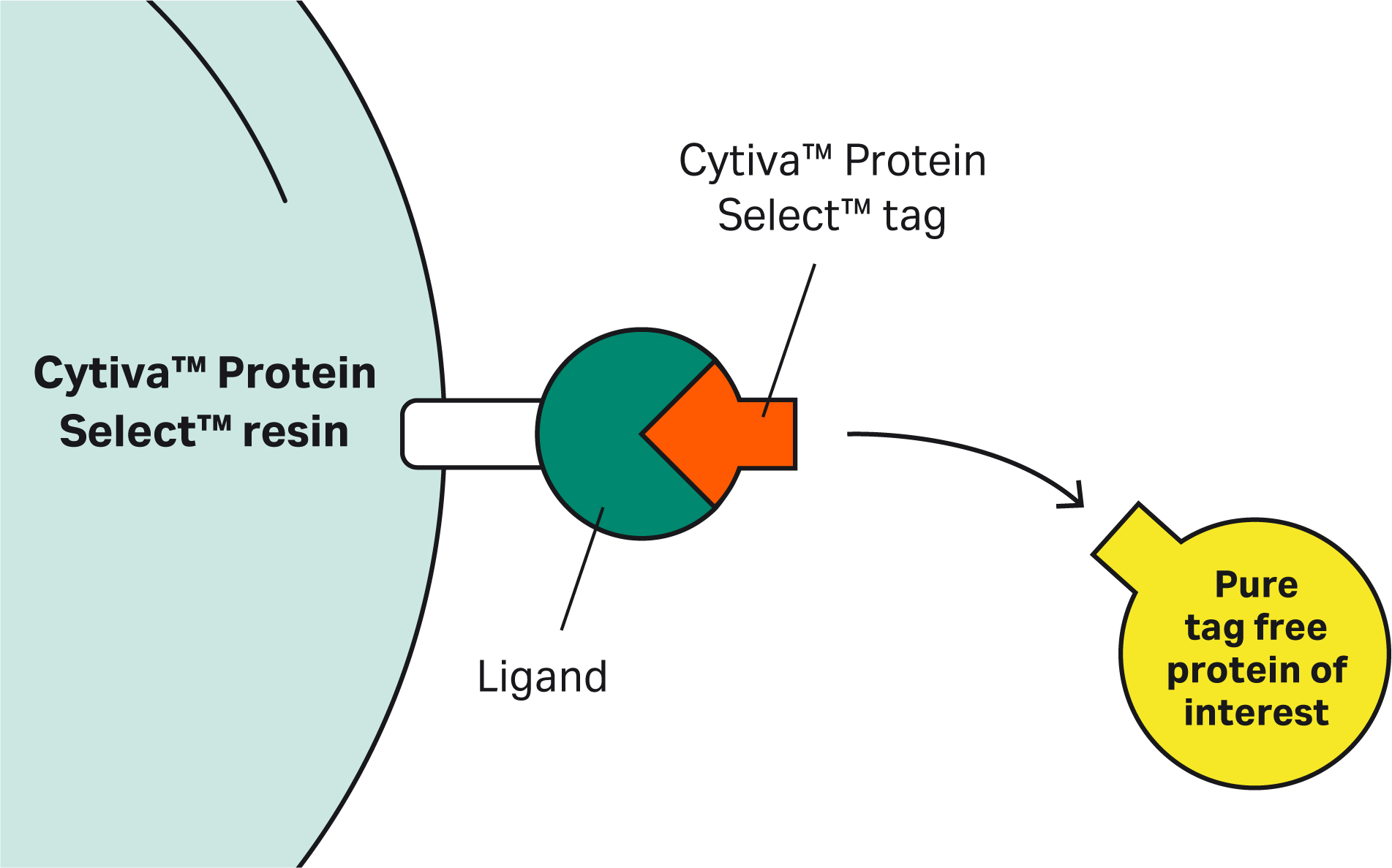
With questions covering a range of topics from principles to protocols, we’ve heard from scientists who are interested in learning more about Cytiva™ Protein Select™ technology. Their questions and our answers are collected here — scroll through them and you might find the answers you need.
Click on the + sign of each topic below to expand the related FAQs
Want to discuss the Cytiva™ Protein Select™ system? Contact us!
| Question | Answer | |
|---|---|---|
| 1 | Are there any reagents that will prevent purification if they are present in the sample? | Denaturing substances will prevent purification. Other than that, we’ve tested many common buffers, salts and excipients, including EDTA, DTT, and divalent metal ions, and we haven’t seen purification problems with them. |
| 2 | Is it possible to purify samples that contain detergents — specifically CHAPS, NP-40, and detergents for membrane proteins? | The presence of non-denaturing detergents is probably fine. We used CelLytic™ Express (Sigma-Aldrich) for extraction of target proteins produced intracellularly in E. coli, and subsequently did purification with success. The kit does not say exactly what the detergents are but mentions: a blend of detergent and enzymes. But we have not yet purified a membrane protein ourselves. |
| 3 | One of the proteins I want to express with the system would need to be purified totally under denaturing conditions. Can the whole purification (including binding, cleavage, etc.) be done in 4-8 M urea? | No. Purification cannot be run under denaturing conditions. |
| 4 | Our protein has a tendency to oligomerize via S-S bridges. How does Cytiva™ Protein Select™ technology deal with this phenomenon? Is adding antioxidants to the buffer compatible with the technology? | For the excipients, reducing agents or antioxidants and ascorbate would work. |
| 5 | We are working with insoluble protein and therefore using 8 M urea. Would this work with Cytiva™ Protein Select™ resin? | No. 8 M urea in the binding buffer will not work, because the tag needs to bind to the ligand on the resin and the complex needs to fold, which will be difficult to achieve in the presence of 8 M urea. |
| 6 | Is the resin compatible with buffers containing amino acids, for example 50 mM to 1 M arginine (used to prevent aggregation) or 10 mM histidine buffer? | Probably. We haven’t tested this, but based on what we know, the resin should be compatible with amino acid buffers. A pH between 6 and 9 and ionic strength corresponding to 0-1 M NaCl works. The ligand tolerates high concentrations of urea (>4 M) in 50-100 mM NaOH during regeneration/CIP. |
| 7 | Can the eluate from an immobilized metal chelate affinity chromatography (IMAC) purification be directly applied to the Cytiva™ Protein Select™ column (i.e., without removing imidazole)? | Yes. You can apply samples containing imidazole to Cytiva™ Protein Select™ resin. |
| 8 | Is it possible to use the tag system in the presence of reducing agents? | Yes, you can use reducing agents (e.g., DTT) during purification. |
| Question | Answer | |
|---|---|---|
| 1 | What do you think of using Cytiva™ Protein Select™ in food applications such as enzyme purification? | Cytiva™ Protein Select™ resin can be used for enzyme purification in food applications. More information will be provided (together with the regulatory support file (RSF)) in the near future. |
| Question | Answer | |
|---|---|---|
| 1 | What is the binding capacity of Cytiva™ Protein Select™ resin? | Since the tagged protein will cleave once bound to the resin, it is not possible to measure the traditional dynamic binding capacity (QB10) using UV signal. Yield will depend on protein design and cleavage time (hold time). We have measured yields (the amount of cleaved target protein in the eluate) up to 20 mg protein per mL resin. |
| Question | Answer | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | What is the mechanism of self-cleavage? |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
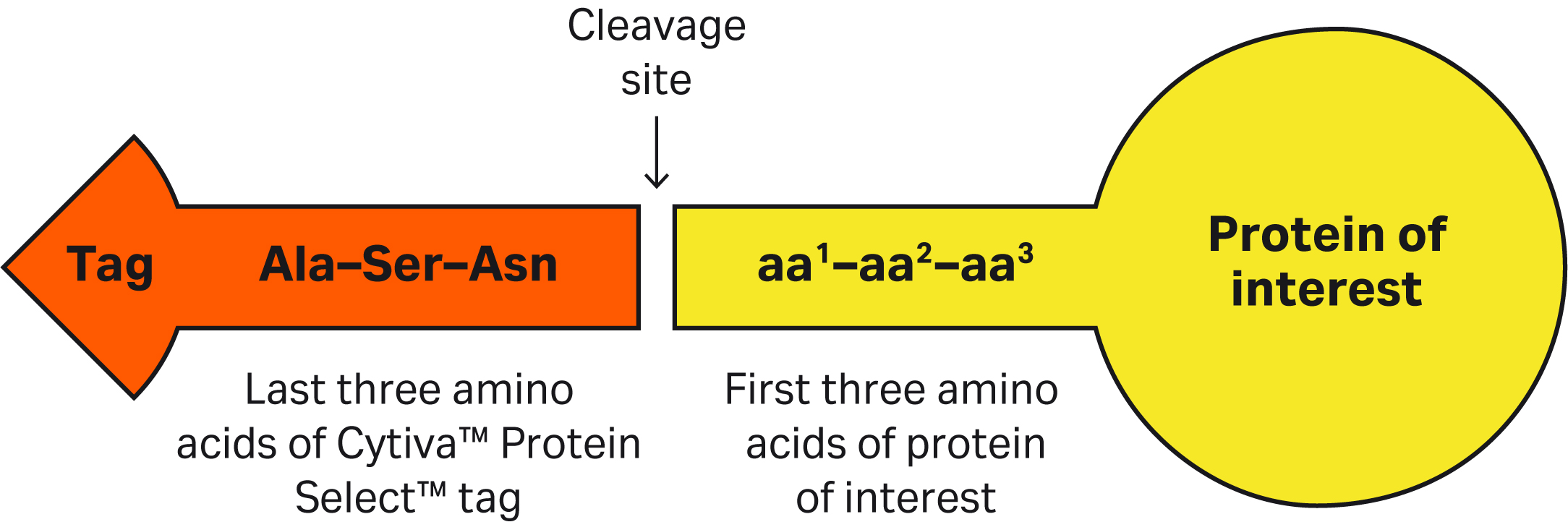
| 2 | How do the first three amino acids at the N-terminus affect the cleavage kinetics and cleavage time for proteins? | Hydrophobic and aromatic amino acids in the second position will increase the rate of cleavage. Small, nonpolar, or negatively charged amino acids in the first position will decrease the rate of cleavage. Proline amino acids located in first and/or second position halts the cleavage. The cleavage table show the expected cleavage rates for different combinations of the first two N-terminal amino acids of the target protein. Combinations labeled with a plus sign (+) have a faster cleavage rate than combinations labeled with a minus sign (-). The amino acid at the third position also affects cleavage rate, although to a lesser extent. It is the combined properties of the first three amino acids that is defining the cleavage kinetics. For proteins whose first 3 amino acids are expected to have a slow cleavage rate, one might consider mutating some of these amino acids or inserting extra amino acids at the N-terminus to increase the cleavage rate. Inserting Ala-Phe-Val or Phe-Arg-Val at the N-terminus has shown fast cleavage for the proteins we have tested. The duration of the hold step is protein dependent. Four hours is recommended for the first trial. Overnight incubations may be required for slow-cleaving targets, especially if cleavage is performed at lower temperatures.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
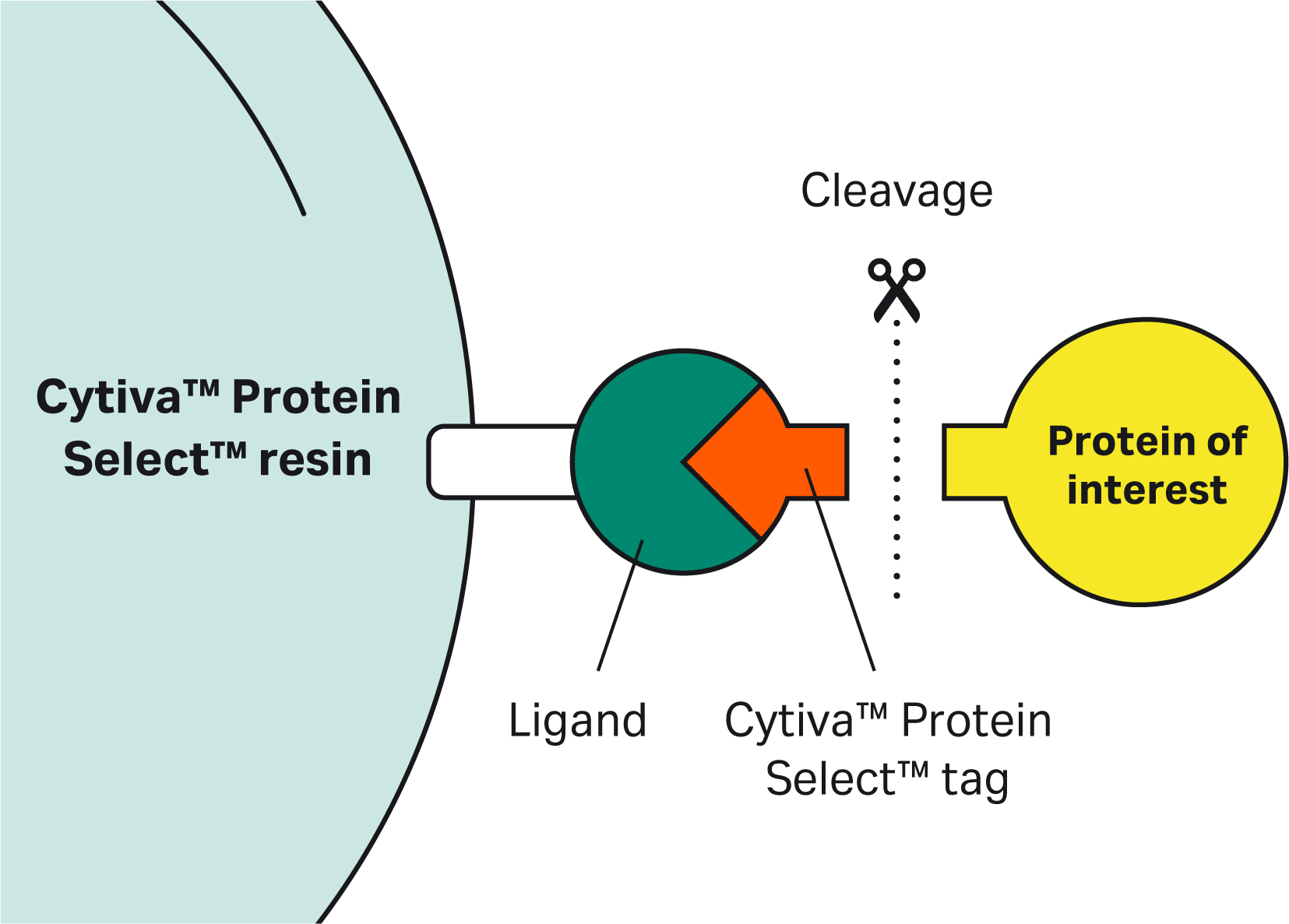
| 3 | What initiates the cleavage? | The cleavage occurs after the tagged protein binds to the resin. The reaction is spontaneous and does not require additional excipients or stimuli. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4 | Does the self-cleavage require reducing conditions or any special condition? | No, the cleavage reaction is spontaneous and does not require additional excipients or stimuli. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5 | Does cleavage occur only after the sample loading and wash step? | No, The cleavage will start as soon as the tagged protein is bound to the resin. The instructions recommend a maximum of 30 min sample loading and wash time, but one should do sample loading and wash as fast as possible to minimize loss of target protein. If you have a slow-cleaving protein, you might still have reasonable recovery with sample loading and wash lasting longer than 30 min. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6 | What is cleaving the tag? Do we need to use special reagents or buffer while the tagged protein is bound on the resin? | No, you do not need special buffers or reagents for the purification process. The tag is self-cleaving, and the cleavage reaction is spontaneous and does not require additional excipients or stimuli. You can use one buffer for the whole purification process.
Suggested buffers:
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
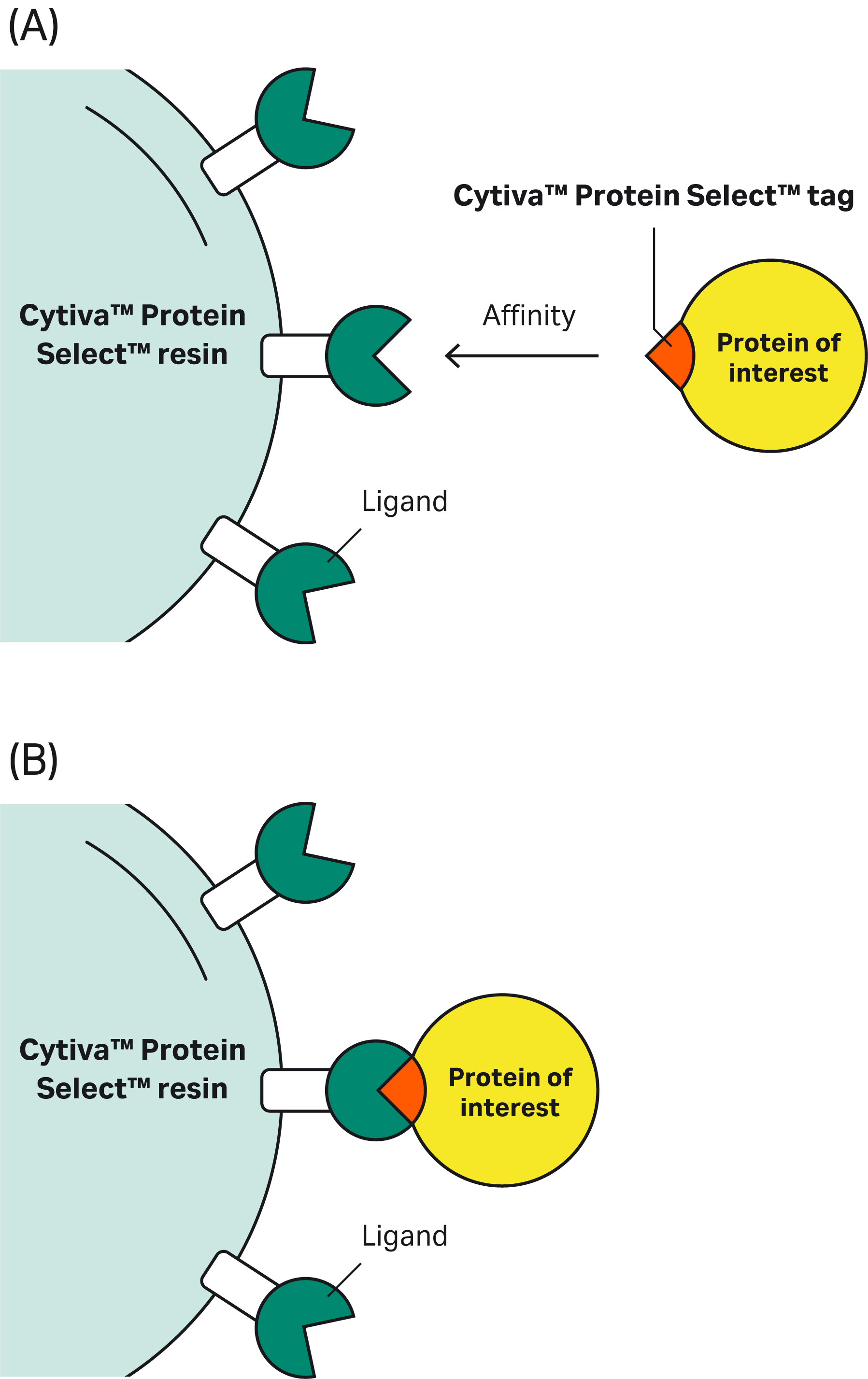
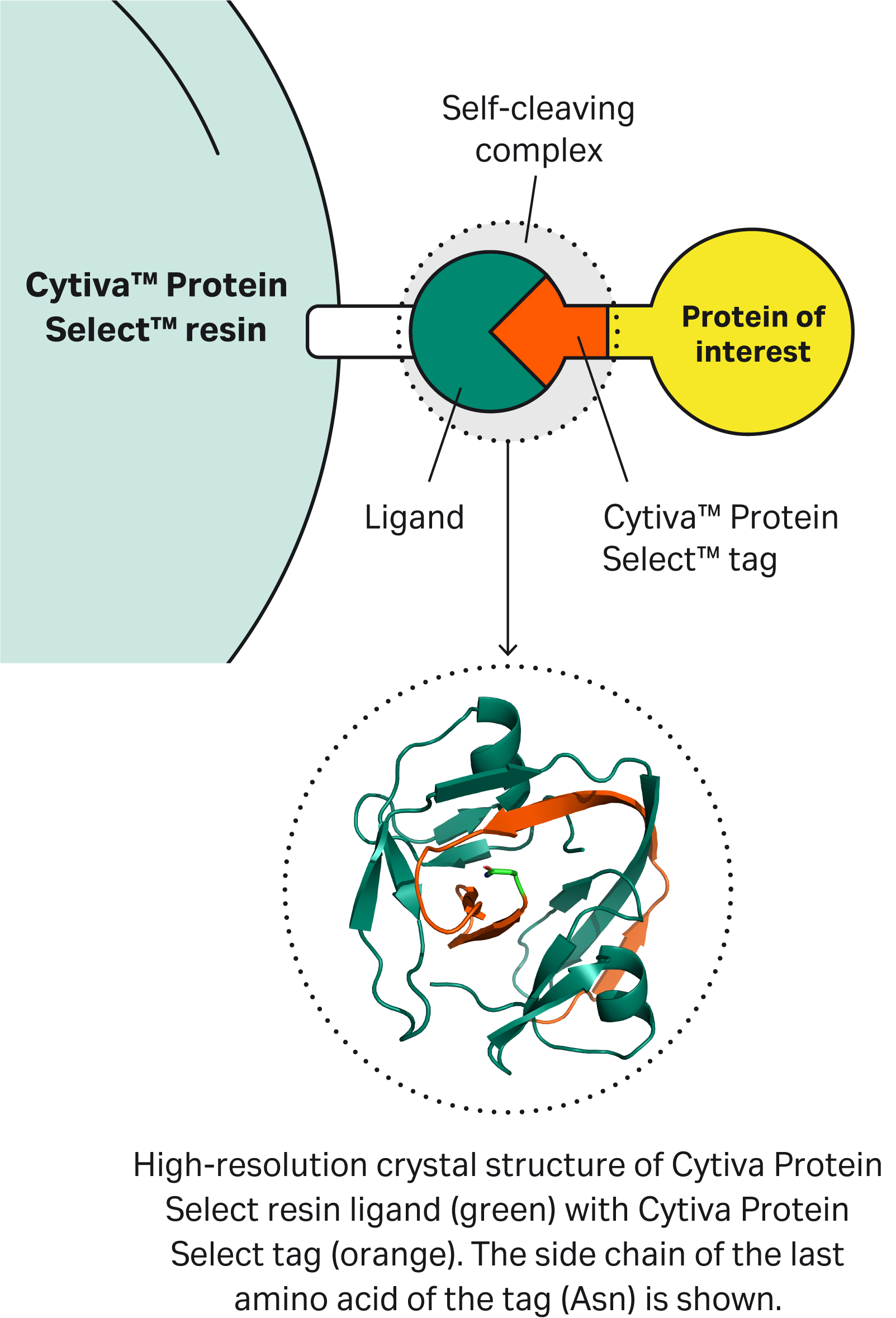
| 7 | Why doesn’t the Cytiva™ Protein Select™ tag work on the C-terminus? | The tag binds to the ligand on the resin, then the tag-ligand complex folds and forms a self-cleaving complex. Cleavage occurs between the last amino acid of the tag and the first N-terminal amino acid of the target protein. This process cannot happen if the tag is at the C-terminus of the target protein. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8 | Is there protease linked to the column? | No, there is no protease involved in the system. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 9 | How do post-translational modifications (PTMs) affect the cleavage yield? | PTMs can potentially affect the cleavage rate. In our experiments with purification of the glycosylated receptor-binding domain (RBD) of the SARS-CoV-2 spike protein, the protein cleaved as expected. See data file. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Question | Answer | |
|---|---|---|
| 1 | Can the resin be used in GMP production? | In the future, support and products for scaling-up to clinical and commercial scale will be available including regulatory support files and ligand leakage kit. Larger resin containers (1 L, 5 L, and 10 L) will eventually be available for packing in larger columns, such as HiScale™ or AxiChrom™ chromatography columns. |
| Question | Answer | |
|---|---|---|
| 1 | Will there be a licensing fee for using the product? | No, there will not be any license or royalty fee for using this product. |
| Question | Answer | |
|---|---|---|
| 1 | What is the nature of the ligand? Is it a small molecule, peptide, something else? | Cytiva™ Protein Select™ technology uses a protein ligand produced in E. coli. |
| Question | Answer | |
|---|---|---|
| 1 | What is the ligand leaching detection method for Cytiva™ Protein Select™ resin? | More information on ligand leaching (including method for testing) will be provided (together with RSF) in the near future. |
| 2 | Do you have a toxicity study on the ligand and the tag? | No, but we have done a toxicity risk assessment. The results so far suggest that the ligand and the tag are unlikely to have toxic effects. |
| 3 | Will there be an ELISA kit both for the ligand and the tag? | More information on ligand leakage, ligand toxicity, etc., will be provided (together with RSF) in the near future. |
| Question | Answer | |
|---|---|---|
| 1 | Will a higher-molecular weight solubility tag (GST or MBP) in front of the Cytiva™ Protein Select™ tag influence the interaction between the Protein Select™ tag and the ligand on the resin? That is, is it necessary to remove the added tag before binding to the ligand? | Although we have not tested this, it should be OK to have an MBP or GST tag upstream of the Cytiva™ Protein Select™ tag. It should still bind and cleave (a potential issue with GST is however its propensity to dimerize, which would affect the cleavage). You might also consider adding a linker (several amino acids) between the two tags to reduce the risk of steric hindrance. |
| 2 | Regarding adding a signal peptide or dual tag upstream of the Cytiva™ Protein Select™ tag, is there a limit to the size? Could you add a sequence of 90-100 aa, for example? And how would that affect binding or cleavage efficiency? | We are not aware of a size limitation of a dual tag or a signal peptide upstream of the Cytiva™ Protein Select™ tag. Things to consider are potential interactions of a dual tag and a suitable linker between the tags to allow a certain degree of movement for the different parts of the fusion protein. Any effects of an additional tag on binding and cleavage could be tested against a single-tagged control protein. We have seen minor effects on cleavage rate (slightly slower), with an additional 40 aa affinity tag attached via a (GGSG)-linker. |
| 3 | Can a small tag, such as a His- or FLAG tag, be cloned between the Cytiva™ Protein Select™ tag and the target protein? | We have tested proteins with His-, Strep-tag® II, and FLAG tags upstream of the Cytiva™ Protein Select™ tag. They bind to the resin and cleave as expected. If the additional tag is placed between the target protein and the Cytiva™ Protein Select™ tag, the system will try to cut between the Cytiva™ Protein Select™ tag and the additional tag. The target protein will still have the additional tag in this case. In contrast, if the additional tag is placed upstream of the Cytiva™ Protein Select™ tag, this would still generate tagless target protein (i.e., the system tag is always cut at the C-terminus). *Strep-tag is a trademark of IBA GmbH. |
| 4 | Do you think the Cytiva™ Protein Select™ tag could help solubilize the protein? | The tag is rather small (about 4 kDa), so it will not help solubilization. You can place a solubilization tag upstream of the Cytiva™ Protein Select™ tag. You might also consider adding some linker amino acids between the tags. |
| 5 | Will adding a FLAG or histidine tag upstream of the Cytiva™ Protein Select™ tag prevent or affect capture by the ligand? | No. It is OK to have a FLAG or histidine tag upstream of the Cytiva™ Protein Select™ tag. We have tested proteins with other tags upstream of the Cytiva™ Protein Select™ tag and they worked well. |
| Question | Answer | |
|---|---|---|
| 1 | Does the cleavage start within the expression system? | No, it does not. Cleavage starts only after your target protein is bound to the resin (after which a self-cleaving complex is formed). Cleavage will not occur during expression or sample preparation. |
| Question | Answer | |
|---|---|---|
| 1 | Are there any sequences that can serve as positive or negative controls? | Yes. We have been using human IL-1ß for positive and negative controls. The mature sequence contains a proline in the second position, which binds but cleaves poorly, so it can be used as negative control. The positive control has a proline-to-phenylalanine point mutation, which cleaves relatively fast. |
| 2 | If the tag sequence is being used in a mAb, does it matter if it is on the light chain or heavy chain? | This must be empirically tested. Note also that the kinetics of the cleavage reaction are likely to be different from that of monomeric proteins and will likely need some optimization. |
| 3 | What is the impact of free cysteine? | Addition of cysteines to the media or the protein of interest should not impact binding and cleavage activity. |
| 4 | You observed that hydrophobic/aromatic amino acids in the second position may increase the rate of cleavage. Is this increase enough to cause a problem — for example, loss of a lot of target protein in the wash due to the speed of the reaction? | It could happen, especially during a lengthy sample application. An optimization of the purification method would be needed to increase the yield. For example: screening for conditions (pH, salt, temperature) that minimize cleavage rate and/or a concentration of the sample to reduce the overall loading/wash time. |
| 5 | Do you have data on how this works with multimeric proteins? I am worried about the fact that the tag is on the N-terminus, which is the region involved in multimerization. | We don’t have much data on multimeric proteins. The effects are probably dependent on the nature of the protein, i.e., whether it is a homo- or hetero- multimer, whether all monomers are tagged, etc.
If the protein is a hetero-dimer and only one monomer is tagged, for example, the process should work. |
| 6 | You mentioned the importance of amino acids in positions 1 and 2. What about the effect of the amino acid at position 3 at the N-terminus of the target protein? | Position 2 has the largest impact on cleavage rate, followed by position 1. Position 3 also has an impact, but to a lesser extent — i.e., position 3 is not equally important to consider.
That said, it is the combined properties of the three amino acids (positions 1-3) that define the cleavage kinetics. |
| 7 | Will the performance of the Cytiva™ Protein Select™ resin be hindered with a 60 kDa protein of interest? | No, a protein of 60 kDa will not hinder the performance of the resin. |
| 8 | If the first 3 amino acids of target protein contain sequences that might halt or slow the cleavage process and we don’t want to mutate the target protein, can we add a linker sequence (that includes all the recommended amino acids for efficient cleavage) between the C-terminus of the tag and the N-terminus of the protein? | Yes, one can insert amino acids between the C-terminus of the Cytiva™ Protein Select™ tag and N-terminal of protein. Cleavage will be after the last amino acid of the tag (Asn). Inserting Ala-Phe-Val or Phe-Arg-Val has shown fast cleavage for the proteins we have tested. |
| 9 | The Cytiva™ Protein Select™ tag ends with Ala-Ser-Asn. What happens if we have the same sequence at or close to the N-terminus of our protein? Would we see unexpected cleavage? | Having an Ala-Ser-Asn sequence in your protein will not affect the cleavage position. After the tag binds to the ligand on the resin, the tag-ligand complex folds and forms a self-cleaving complex, wherein cleavage occurs after Asn of the Ala-Ser-Asn in the tag. |
| 10 | Is there any limitation regarding the use of further tags upstream of the Cytiva™ Protein Select™ tag? For example, how does size play a role (e.g., MBP tag)? Any recommendation here? Which tags have worked best so far? | We have mainly tested epitope tags like polyhistidine, FLAG-tags, and Twin-Strep-tag®. The main effects we have seen from using additional tags upstream of the Cytiva™ Protein Select™ tag are slightly slower cleavage kinetics, larger mass-shifts on SDS-PAGE (makes it easier to see the cleavage), and some protection from proteolytic cleavage for some test proteins. *Twin-Strep-tag is a trademark of IBA GmbH. |
| 11 | If we have some small and nonpolar or negatively charged amino acids in the first, second, and third position of the protein, will the cleavage be total after 24 h? | No, some uncleaved protein will likely remain bound to the resin. If you want to improve the cleavage kinetics, you could also consider adding two additional amino acids (such as Ser-Leu). We have also tested for predicted protein domains and shifted the start of the domain (e.g., +1 or -1 from the intended start). In this way the sequence will still be "native". |
| 12 | Does the size of the target protein affect the binding or cleavage efficiency? | The first three amino acids have a larger impact than the size of the protein. Very large proteins could potentially have sterical hindrance during binding to the resin. |
| 13 | Does the system work with PEGylated proteins? | It should work with PEGylated proteins, but it's not something we have explored. |
| 14 | What are the protein sizes tested so far? | We have tested proteins between 15 and 80 kDa. |
| 15 | Your data file says the first 3 amino acids of the protein are critical for self-cleavage efficiency. Do you have examples of the most optimal amino acid sequences? | In our tests, proteins that have Ala-Phe-Val or Phe-Arg-Val as the first 3 amino acids showed fast cleavage rate. |
| Question | Answer | |
|---|---|---|
| 1 | The data file says that 1 M is the highest NaCl concentration recommended for the buffer; what is the lowest NaCl concentration recommended? | Salt is not required for binding or cleavage, but a good starting point could be to add 150-300 mM NaCl to minimize unspecific interactions and increase purity. |
| 2 | Do you have experience using this affinity tag and resin with proteins at low expression levels — for example, 100 mg/L and a 200 L scale? We're wondering about loading time and the risk of losing protein of interest at flow-through. | Cytiva™ Protein Select™ needs a shorter sample load and washing phase because of the self-cleaving activity, so it’s best to avoid low-expressed proteins unless you can add a concentration step before binding.
For example, you could concentrate mammalian cell feeds by ultra-filtration to increase sample load. Concentration can also be done using other techniques than ultra-filtration, including ion-exchange chromatography. |
| 3 | You recommend 4 h or overnight for the hold step. What would happen if we were to leave it 1 or 2 days? | Most of the cleavage reaction occurs at the beginning of the hold step, so a hold time of 1 or 2 days can increase cleavage, but the size of the effect depends on the protein. The outcome would also depend on whether the target protein is stable during that time. |
| 4 | At 50°C or 60°C, is it possible to have efficient self-cleavage? | We don’t have data on cleavage at 50°C or 60°C. Theoretically, cleaving is faster at these temperatures, but one has to consider the stability of the column hardware, resin, and the target protein. |
| 5 | Do you have any recommendations for batch purification? | Because tag cleavage occurs after the tagged protein is bound to the resin, we recommend fast sample application and wash. Batch purification is slower than running over a packed column and can potentially cause more loss of target protein. That is why we recommend purification using a packed column. |
| 6 | Is the sample loading and wash time limited to a maximum of 30 min? | The 30 min sample loading and wash time is a guideline to minimize risk of losses. However, the maximum loading and wash time depends on the cleavage kinetics of your target molecule. The impact of loading time is lower for a slow-cleaving protein. |
| 7 | What is the yield in percent? | The percent yield depends on several factors, including the first three amino acids of the target protein (which affect cleavage rate), the sample loading and washing time, and the hold time. |
| 8 | What sample preparation method should I use for a target protein expressed in E. coli? | We typically recommend methods and techniques described in Chapter 6 of the handbook Strategies for protein purification. |
| 9 | The buffer recommended for Cytiva™ Protein Select™ resin is MES, Tris or PBS with pH 6-9, but how about adding low-concentration salt to the buffer to reduce nonspecific reactions, especially during sample application? | It is always good to have some salt to reduce nonspecific reactions. We recommend 20-50 mM buffer compound supplemented with up to 1 M salt (preferably NaCl), at a pH range of 6 to 9, which means one can use 0 to 1 M salt. |
| 10 | How efficiently does the tag bind to the ligand during sample application? At the maximum recommended sample application of ~600 cm/h, is it possible that some of the sample might end up in the flowthrough? | The affinity between the tag and the ligand is very high, and we see fast binding of tagged protein to the resin. A small amount of cleaved protein will be in the flowthrough and wash (this is also dependent on the first three amino acids of the protein, which affect the speed of cleavage). |
| 11 | If I purify protein from cell culture supernatant with a large sample volume and sample loading takes a long time, is there a risk that parts of the protein will be cleaved and elute before loading of the column is complete? | We recommended that sample application including wash step are completed within 30 minutes. This is because if the target protein is fast cleaving, some loss of cleaved protein will occur during sample application. Consider sample concentration before purification. |
| 12 | Can we perform the purification at 4°C? | Yes, you can run the purification at 4°C (such as in a cold room) but cleavage will be slower, so you may need to increase the time for the hold step. |
| 13 | Why do the His-tagged and Cytiva™ Protein Select™ tagged proteins look like they’re different sizes after purification (on the SDS-PAGE gel shown in the data file)? | In the data file we showed a comparison of Cytiva™ Protein Select™ tag vs His-tag with TEV protease. The purified protein from the Cytiva™ Protein Select™ tag does not contain extra amino acids, while the one from His-tagged protein contains residual amino acids after TEV cleavage. We confirmed both molecular weights by LC-MS. See data file. |
| 14 | How do you prevent tag cleavage during the binding and wash steps? | The cleavage will start once the tagged protein is bound to the resin. This is why we recommend the sample loading and wash are done as fast as possible. |
| 15 | What is the composition of the elution buffer? Is it necessary to exchange elution buffer for dialysis or size exclusion chromatography, for example? | The protein will elute in the buffer that was used for wash after sample loading. There is not a dedicated elution buffer. |
| 16 | Can wash buffers be applied to remove unwanted impurities? | Yes, after sample loading you can use different buffer(s) for washing out unwanted impurities. |
| 17 | Can we use protease inhibitors in buffers? | Yes. Pefabloc® and EDTA worked well in our purification. |
| 18 | What is the pH dependency of the cleavage reaction? | The cleavage is rather insensitive to pH at range 6-9. For some proteins, switching from, say, MES pH 6.2 buffer for sample loading to Tris pH 8.5 for washing buffer might increase the cleavage rate, but this is of course protein dependent. |
| 19 | Can you comment about nonspecific binding on the resin? Do we eliminate polishing step? | We have not seen any particular issue with nonspecific binding, but it is always a good idea to include some salt in the buffer to reduce nonspecific binding. |
| 20 | The compatibility table in the data file gives very low concentrations for common buffers (e.g., 10 mM for HEPES and phosphate). Is that an upper limit, or could a higher concentration of these buffers (e.g., 50 mM) be used? | The table in the data file lists the concentrations we have tested. That said, it should be fine to use, e.g., 50 mM phosphate buffer. |
| 21 | Does cleaving rate impact the protein purity? | No, cleaving rate does not impact purity of eluted protein. |
| 22 | How do you prove traceless cleavage? | The eluted protein has no traces of tag amino acids, confirmed by mass spectrometry analysis. |
| Question | Answer | |
|---|---|---|
| 1 | In the data file you showed mass spec data on the scaffold protein. Have you characterized the analysis artifact peak? | Yes, it is an acetonitrile adduct (+42) and likely a neutral loss of H20 (-18). |
| Question | Answer | |
|---|---|---|
| 1 | What is the base matrix of the resin and why was this base matrix chosen? | The resin is based on an established high-flow agarose matrix with a particle size of ~ 60 μm. This is one of Cytiva’s modern affinity purification resins. We chose it based on its resolution and scale up capabilities. |
| 2 | What are the formats available for Cytiva™ Protein Select™ resin? | Cytiva™ Protein Select™ resin is available in 25 mL, 100 mL, and 500 mL packs of bulk resin and in HiTrap™ 1 mL and 5 mL columns (HiTrap™ Protein Select™ columns), which are well suited for research and process development work. Instructions are supplied for packing Cytiva™ Protein Select™ resin in Tricorn™ columns. You can see all formats on the Cytiva™ Protein Select™ shop page. In the future, support and products for scaling up to clinical and commercial scale will be available, including regulatory support files (RSFs) and ligand leakage kits. Larger resin containers (1 L, 5 L, and 10 L) will eventually be available for packing in larger columns, such as HiScale™ or AxiChrom™ chromatography columns. |
| Question | Answer | |
|---|---|---|
| 1 | How do I regenerate the resin? | To effectively regenerate/CIP the resin between purification cycles, we recommend using 4 M urea with 100 mM NaOH or 4 M guanidine hydrochloride with 100 mM NaOH. |
| 2 | Is it okay to regenerate the resin only with denaturants or NaOH? | No. 4 M urea alone or 100 mM NaOH alone will NOT effectively regenerate/CIP the resin. To effectively regenerate/CIP the resin between purification cycles, one needs a combination of 4 M urea and 100 mM NaOH. |
| 3 | The data file shows that the resin has 80% of its original binding capacity after 20 purification cycles and regeneration and CIP. What is the contact time for the regeneration/CIP step? And what causes the decrease in capacity? | The contact time during regeneration/CIP for E. coli lysate purification was 15 min. The resin was then immediately washed with water followed by a neutral pH buffer to prepare for the next purification. The remaining capacity of 80% after 20 cycles may not be the same for other samples, such as CHO cell culture. Reduction in capacity after regeneration/CIP cycles can be caused by incomplete regeneration (i.e., tag molecules are not removed from the resin during the regeneration/CIP cycle) or damage of the ligand, or a combination of both. |
| 4 | For the sanitization of the resin, it is indicated to use 4 M urea in 0.1 M NaOH. What is the stability of urea at this pH (~12)? | Urea solutions should always be freshly prepared and used, as solutions of urea may develop cyanate ions upon standing, especially at higher temperature. For small-scale purifications, we prepare aliquots of the regeneration solutions and store them at -20°C. |
| 5 | Can you strip the tag and reuse the resin? | Yes. Regeneration and CIP is possible with a combination of chaotropic agents (urea or guanidine-HCl) and NaOH. |
| 6 | How many times can the resin be reused? | We tested 20 purification cycles using E. coli lysates, after which the resin retained 80% of its binding capacity. See the data file for more information. |
| Question | Answer | |
|---|---|---|
| 1 | Can any signal peptide be used in combination with the Cytiva™ Protein Select™ tag? Other than removing the initial methionine of the tag, are there signal peptide constraints or limitations? | In principle, any signal peptide can be used with the Cytiva™ Protein Select™ tag. We have tested just a few. Removal of the tag’s initial methionine is not required. |
| Question | Answer | |
|---|---|---|
| 1 | How big is the Cytiva™ Protein Select™ tag? | The Cytiva™ Protein Select™ tag is roughly 4 kDa in size. It is made of 36 amino acids. For more information about this tag, visit cytiva.com/protein-select, choose your application, and fill in the form. The sequence of the tag will be sent by email to you immediately. |
| 2 | What is the affinity of the Cytiva™ Protein Select™ tag to the resin? | The affinity between the Cytiva™ Protein Select™ tag to the ligand on the resin is very high. Measurements of surface plasmon resonance (SPR) signals on a Biacore™ instrument show very high affinity and an extremely low dissociation rate. |
| 3 | How can I obtain the sequence of Cytiva Protein Select tag? | Visit cytiva.com/protein-select, choose your application, and fill in the form. The sequence of the tag will be sent by email to you immediately. |
| Question | Answer | |
|---|---|---|
| 1 | Have there been instances of the tag cleaving in the cell culture broth (via protease or cofactor)? | Yes, it can happen. In some cases, reducing temperature for storage or reducing the expression temperature helps. |
| 2 | If the tag is self-cleavable, what is the stability of the tag inside prokaryotic and eukaryotic cells? | The self-cleavage can only occur after binding to the resin. Of course there is the risk that the tag itself can be cleaved by other proteases in the sample, e.g., if the sample is stored for an extended time at room temperature. |
| 3 | Can you recommend cell culture conditions? | No, as this will be very dependent on the protein of interest and the expression system. |
| 4 | How do users determine titer of their cell culture when using this technology? | That depends on the protein of interest (POI). We suggest that the titer determination is done on the POI or on additional tags, if used. |
| 5 | What vector type do you suggest for the plasmid? | Any vector should be fine — or at least, we have yet not found any compatibility challenges to date. |
| 6 | Are yeast or insect cell expression systems suitable for the Cytiva™ Protein Select™ system? | We have extensive experience using E. coli and mammalian cell expression systems (CHO and HEK) for this technology, but not yeast or insect cells. We think it is likely that yeast and insect cell expression would be successful, but we haven’t tested it. |
| 7 | Could you share details such as titer and yield about the upstream process? Is there a difference in performance between His tag and Cytiva™ Protein Select™ tag in protein expression? | The answer depends on the expression system and protein. We have tested the expression levels of native proteins and His-, FLAG-, and Strep-tagged proteins in comparison to the Cytiva™ Protein Select™ tag. In those studies, we did not see an impact on expression levels by adding Cytiva™ Protein Select™ tag. |
| 8 | Does the size of the Cytiva™ Protein Select™ tag relative to that of the target protein affect expression or activity? | In the proteins we’ve tested so far, we haven’t seen any major effect of the tag on target protein expression levels in bacterial or mammalian systems, nor on the functionality after purification and tag removal. |
| 9 | Which signal peptides did you use successfully? | We have successfully used Ig kappa, CD36, and IL-2 signal peptide sequences. |
| 10 | Are antibodies to the Cytiva™ Protein Select™ tag available? | No antibodies to the Cytiva™ Protein Select™ tag are available now. They may be available in the future. In the meantime it is possible to add, e.g., a FLAG or His-tag upstream of the Cytiva™ Protein Select™ tag for detection. |
| 11 | Can we insert the tag into any expression plasmid? | Yes, you can add the tag sequence to your protein of interest and in your vector of choice. This can be done in many different ways, such as tag and gene synthesis, cloning of genes or inserts by PCR or restriction digestion, or Gibson assembly. You can also buy synthetic genes from external vendors. |
| 12 | Can you produce tagged protein in cell culture medium containing serum? | Yes. We have tried a few different media formulations, and we prefer serum free because it's less complex. But the system works with cell media that contain serum as well. |
| 13 | Would this work in cell-free systems? | We haven't evaluated that, but in theory it should work in a cell-free system. |
| 14 | Do you provide the DNA sequence for the Cytiva™ Protein Select™ tag? | The optimal DNA sequence will depend on the expression system and should be optimized together with the protein of interest. There are several codon optimization tools available to improve the rate of translation by overcoming limitations associated with host cell codon usage and the abundance of transfer RNA (tRNA). Although we have not seen an impact on the tag’s performance based on DNA sequence, codon optimization has the potential to cause changes in the target protein’s conformation and functionality.
Many external vendors can produce plasmids optimized for yield based on amino acid sequence and expression system. |
| Question | Answer | |
|---|---|---|
| 1 | Do you have viral clearance data? | We will provide more information in the RSF during the next launch of large-scale formats of Cytiva™ Protein Select™ resin. |