By Michelle Sabourin, Paula Ravnikar, Zhou Jiang, Nan Lin, and Peggy Lio
Abstract
Background and novelty
A robust cell line development (CLD) platform relies on a host cell line that exhibits suspension growth with a consistent doubling time of < 24 h. CLD is particularly challenging, because at least two different media are required to support both low and high cell density growth (single-cell cloning and protein production, respectively). The goal of this work was to establish a host cell line with the desired growth characteristics that was also capable of seamlessly switching between cloning and production media.
Experimental approach
A serum-dependent CHO-K1 host cell line was adapted to serum-free suspension culture in chemically defined and animal-derived component-free (ADCF) media. A two-pronged approach was implemented to ensure success in the shortest timeframe possible. One strategy used sequential adaptation to serum removal followed by adaptation to shaking, while the second strategy used simultaneous adaptation. Both strategies incorporated the use of multiple ACF media.
Results and discussion
The resultant GOCHO™ host cell line was grown for an additional 130 generations and has the following key characteristics: it doubles in < 20 h, displays no cell clumping, and readily transitions between cloning media and production media. Subsequent introduction of model monoclonal antibodies (mAbs) into the GOCHO™ host cell line readily yielded stable clones capable of producing 3 to 5 g/L in a non-optimized shake flask, fed-batch process.
Materials and methods
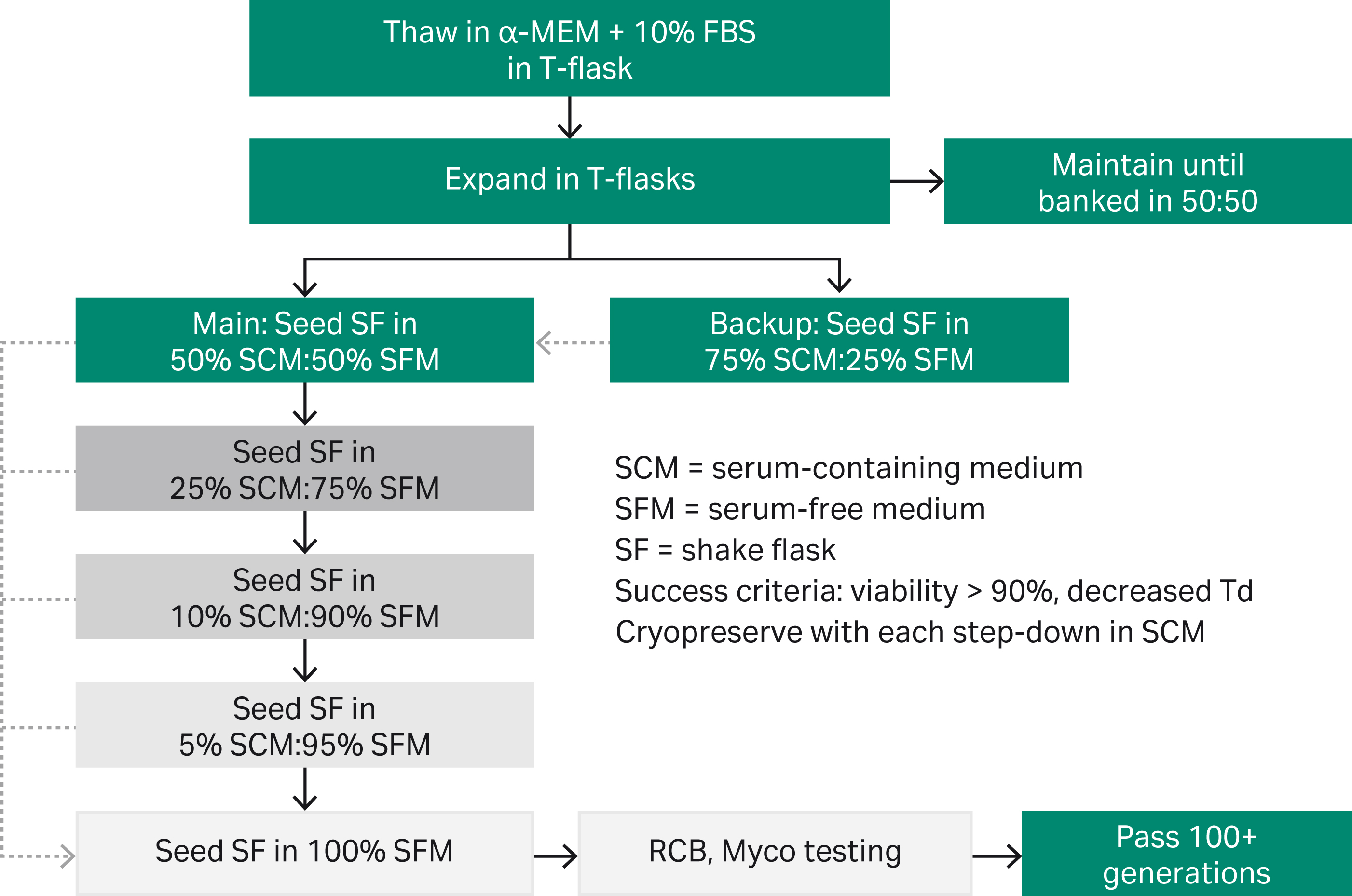
CHO-K1 cells were thawed in a-MEM + 10% fetal bovine serum (FBS; Cytiva). After a brief expansion in T-flasks, cells were seeded in shake flasks (SF) in media mixtures containing 50% of the desired end serum-free medium (SFM; Fig 1). Once success criteria were met at each stage, the cells were transitioned to the next media mixture containing less serum-containing medium (SCM). Cells were cryopreserved at each transition. Once cells were passaged into 100% SFM, a research cell bank (RCB) was generated and tested for mycoplasma. Cells were then thawed and passaged for an additional 100+ generations, with RCBs being generated every ~ 30 generations. Adaptation to multiple HyClone™ media was performed in parallel, with the goal of establishing a cell line that could transition seamlessly between cloning medium and the ultimate production medium, HyClone™ ActiPro™ (SH31039, Cytiva). A seamless transition, defined as maintaining viability > 95% with no apparent growth lag, was tested first in SF (Fig 5), and later within the context of the cell line development workflow; see Figures 6 and 7 for representative clones.
Fig 1. Simultaneous adaptation strategy: CHO-K1 cells thawed in serum in T-flasks were transferred to shake flasks with the first serum reduction step.
Summary
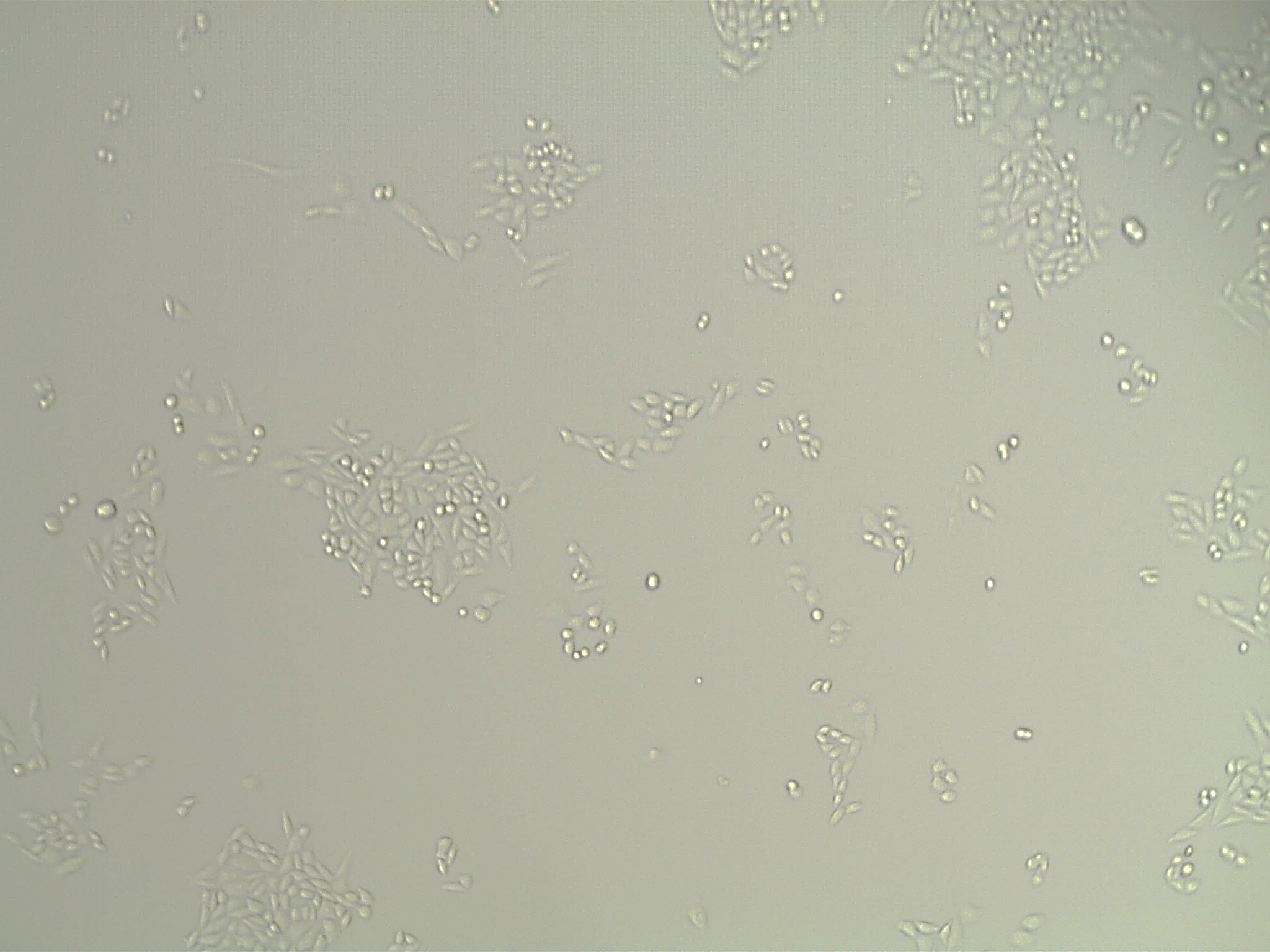
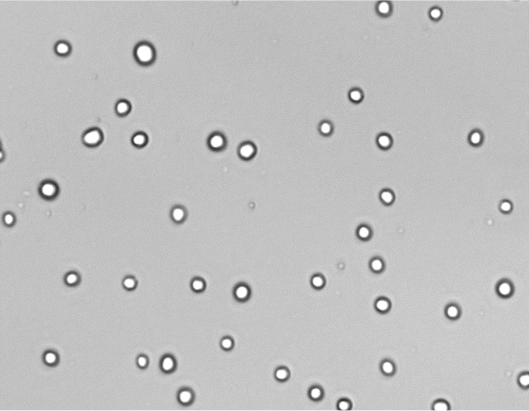
CHO-K1 cells were adapted out of adherent culture in SCM (Fig 2, left) to suspension culture in serum-free, chemically defined medium (right) without clumping.
Fig 2. Images of CHO-K1 pre- (left) and post- (right) adaptation.
Results
Adaptation timeline
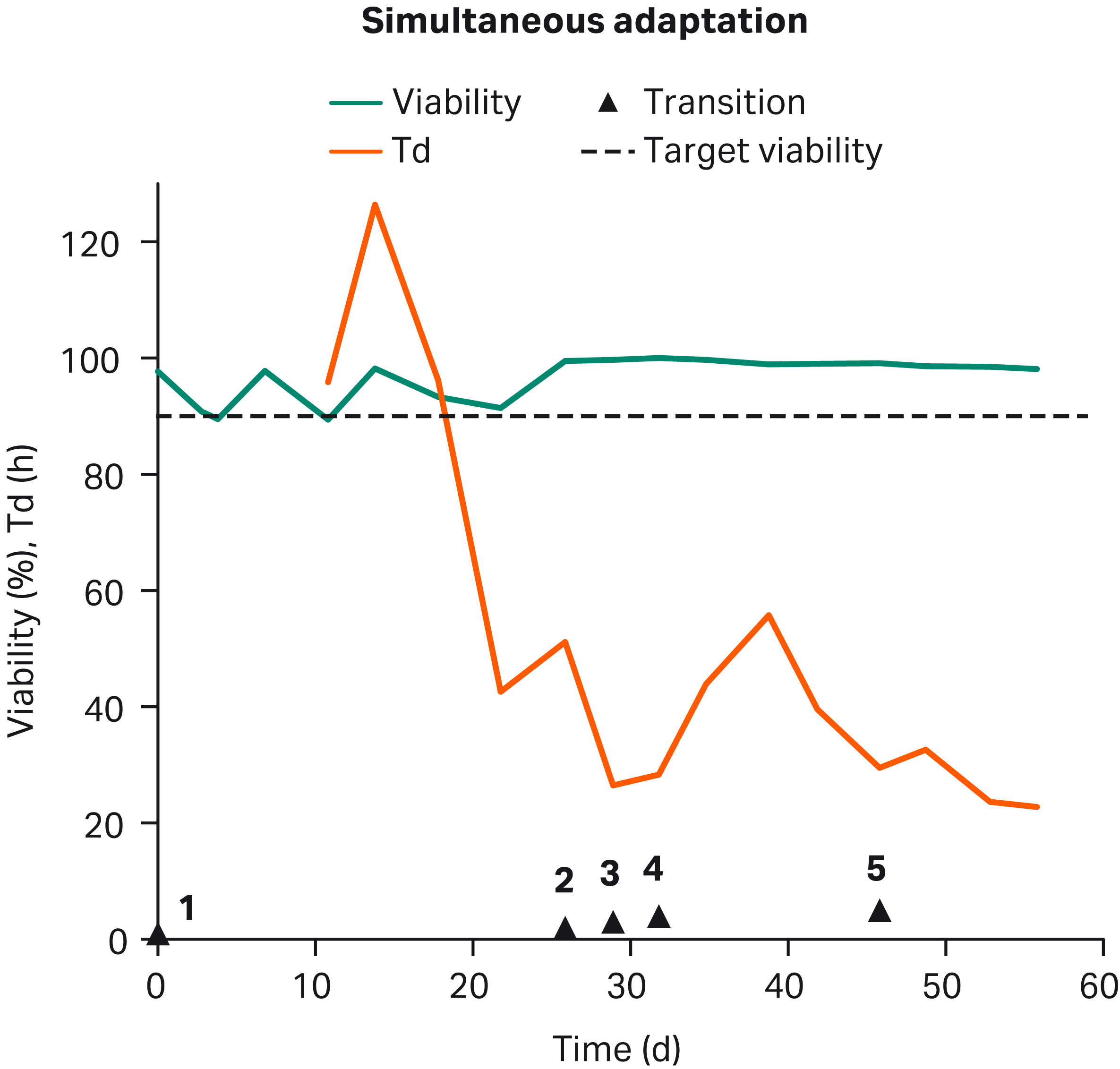
Cells were assessed every 3 to 4 d, and either were passaged in existing medium or into medium with decreased serum per the serum reduction plan (Fig 1). Simultaneous adaptation to both suspension culture and serum-free growth took ~ 8 wk (Fig 3). Additional passage of the GOCHO™ host in SFM for ~ 130 generations decreased Td to < 20 h (Fig 5).
Fig 3. Successful simultaneous adaptation: Adaptation of CHO-K1 to serum-free growth in SF is shown for 1 of the 4 SFM used. After starting in 50:50 SCM:SFM (1), cells were passaged into 25:75 SCM:SFM (2), 10:90 SCM:SFM (3), and 5:95 SCM:SFM (4) before being seeded into 100% SFM (5). Viability (green) remained above the 90% target (purple) from week 3 onward. Td (orange) trended down over time.
Value of multiple parallel adaptations
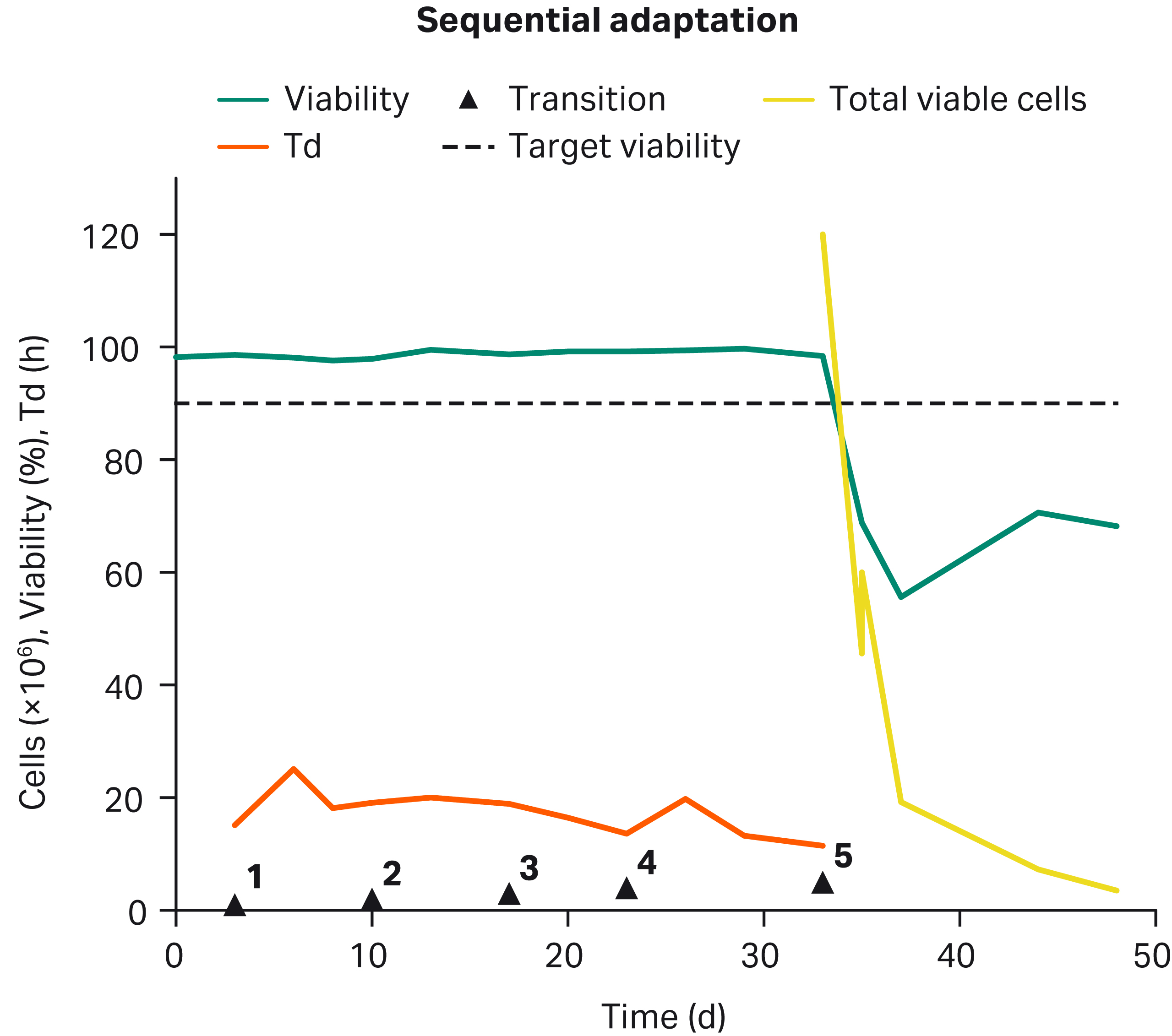
In addition to the simultaneous adaptation strategy, a sequential adaptation strategy was also performed. As Figure 4 shows, after ~ 5 wk to successfully remove serum in T-flasks (transitions 1 to 4), cells failed to transition to SF (transition 5) as indicated by lost growth (negative Td not graphed), loss of viable cells, and no recovery of cell health to > 90% viability.
Fig 4. Failed sequential adaptation: CHO-K1 adapted to serum-free growth in T-flasks failed to transition to suspension growth in SF. After transition 5, total viable cells (yellow) decreased, viability (green) decreased, and Td (orange) went negative (not graphed).
Seamless media switching
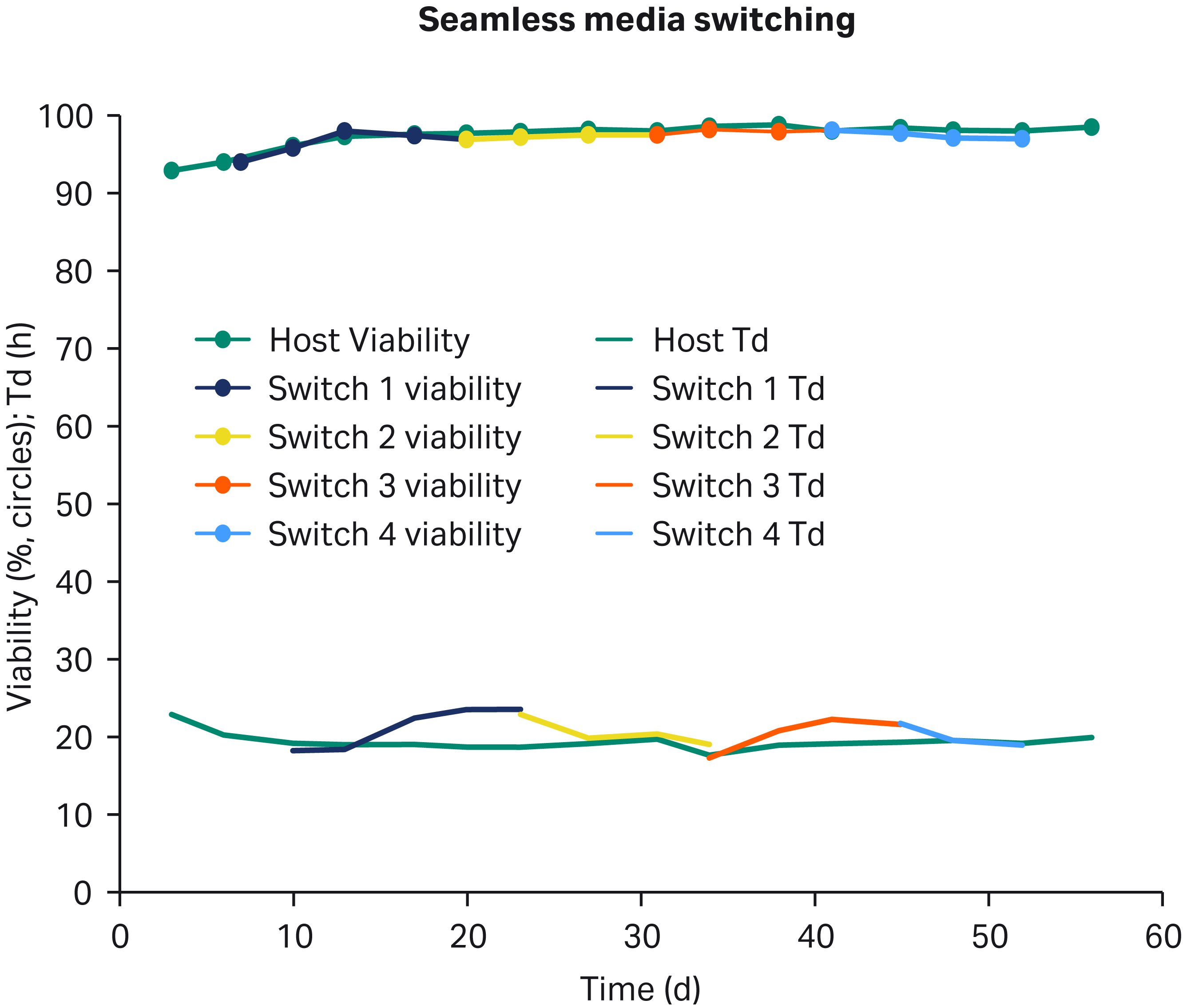
One of the biggest challenges in cell line development (CLD) is the transition in/out of cloning medium. To confirm that GOCHO™ cells were ready for CLD workflow testing, cells were grown in shake flasks and alternately passaged in either cloning medium (CM) or ActiPro™ (AP) production medium (Fig 5). The original train (green) was maintained in parallel for reference. Media switching was observed to be seamless as viability was maintained above 95% and there was no apparent growth lag. The Td of cells in media switching are consistent with the Td of GOCHO™ cells in these media outside of media switching.
Fig 5. Seamless media switching: GOCHO™ cells were thawed and alternately passaged between cloning medium and ActiPro™ medium. Doubling times (Td, bottom lines) and viability (circles) were monitored over 2 mo. Switches: 1 to CM, 2 to AP, 3 back to CM, and 4, back to AP.
CLD workflow testing
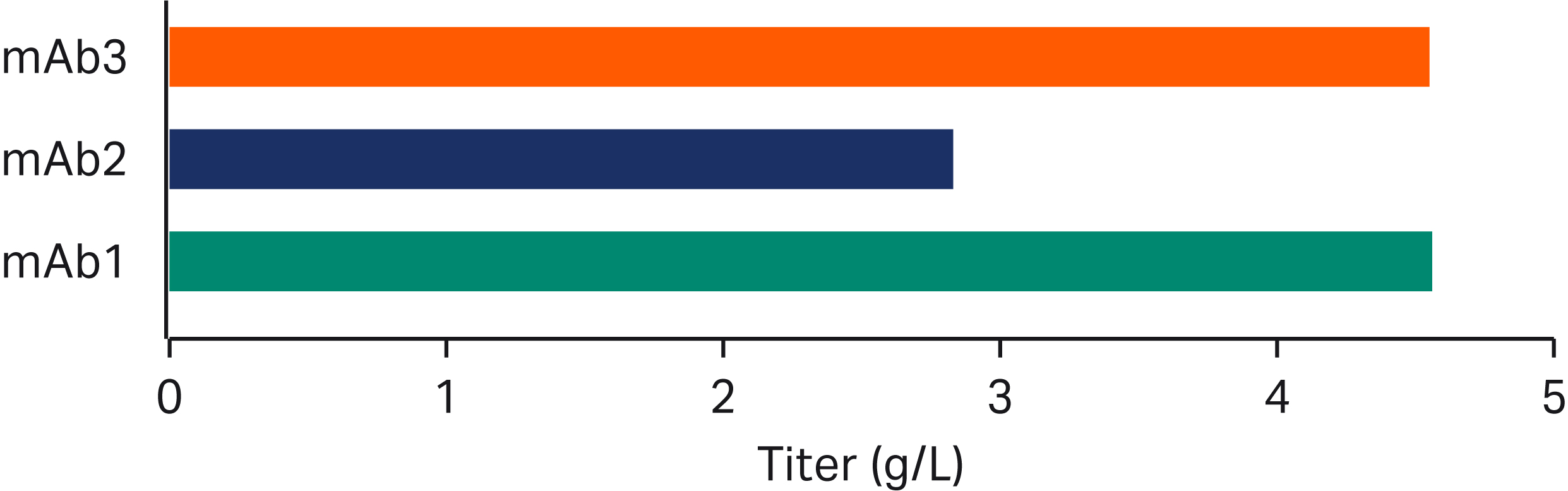
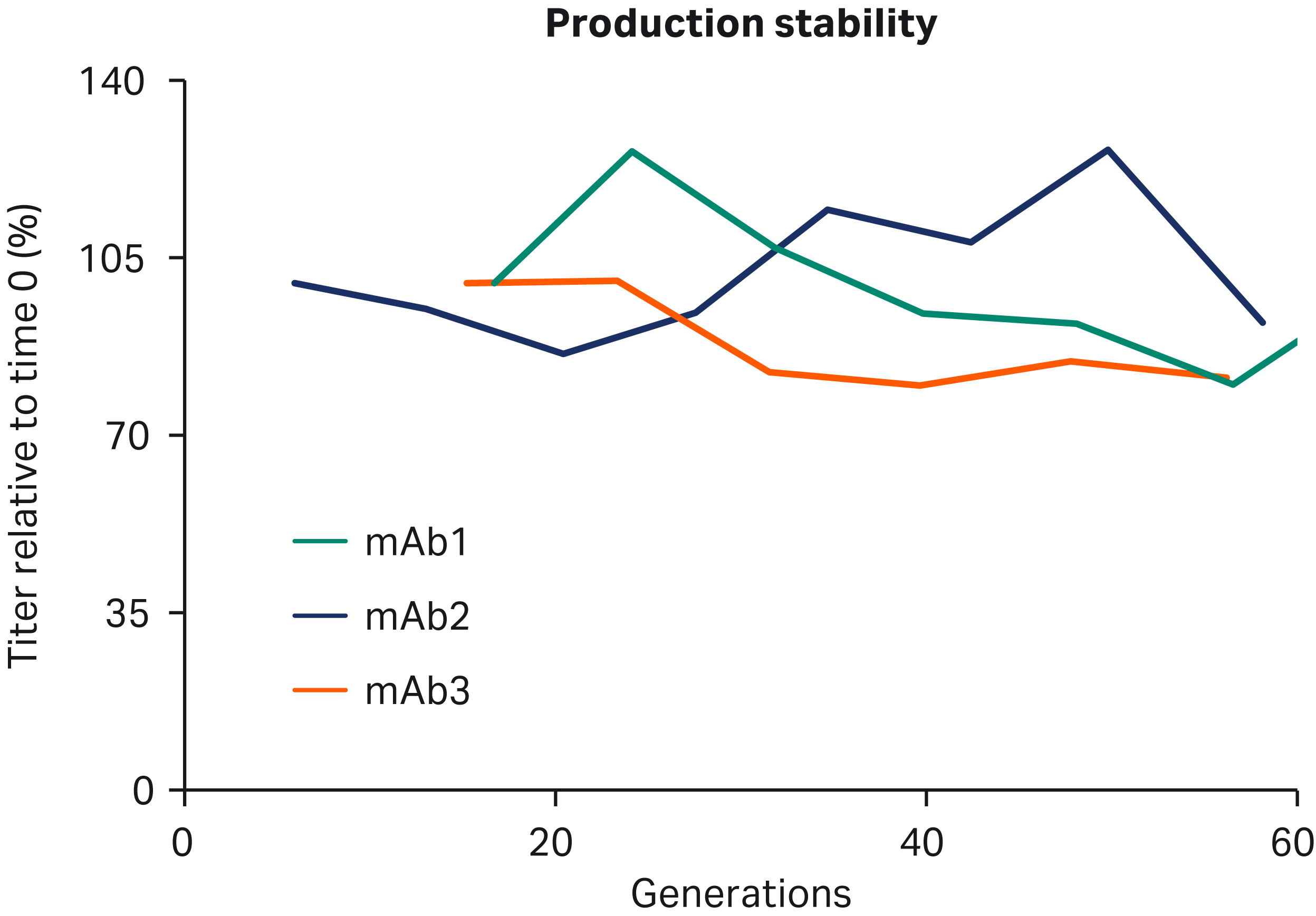
GOCHO™ cells were transfected with three different model monoclonal antibodies, and clones were isolated using cloning medium and ActiPro™ medium in the CLD workflow. Top clones were assessed for fed-batch productivity (Fig 6) and production stability (Fig 7).
Fig 6. Top clone titers: Clones were evaluated for fed-batch titer in SF. The titer of the top clone from each molecule is shown from a standard feed strategy with Cell Boost™ 7a+7b supplements.
Fig 7. Top clone stability: Clones were evaluated for production stability in shake flasks. Weekly productivity assessments were used to establish productivity levels over time, and stable clones were defined as maintaining titers within 30% difference from the initial values.
Conclusions
CHO-K1 cells were successfully adapted out of adherent culture in SCM to suspension culture in SFM (Fig 3). The final GOCHO™ host cell line was free of cell clumps (Fig 2), had a Td of < 20 h, and seamlessly switched between cloning medium and ActiPro™ production medium (Fig 5). GOCHO™ cell performance within the context of the cell line development workflow was confirmed with the successful isolation of stable high-producing clones from three model monoclonal antibodies (Fig 6 and 7).
Learn more about cell line development services and our GOCHO™ platform.