By Tracy Humphries, Cytiva Marketing Leader, Nucleic Acid Therapeutics
Nucleic acid therapeutics are part of the next generation of therapies now taking their place on center stage. Modalities such as viral vectors, RNA forms, plasmids, CAR-T, and cell therapies are increasing in presence in the early clinical stages of therapeutic development. Nucleic acid therapeutics, those based upon RNA or DNA, are offering researchers exciting new avenues of treatment. They can be designed and formulated to silence, express, and edit specific genes, providing a flexible and powerful approach to preventing and treating diseases.
The history of nucleic acid therapeutics
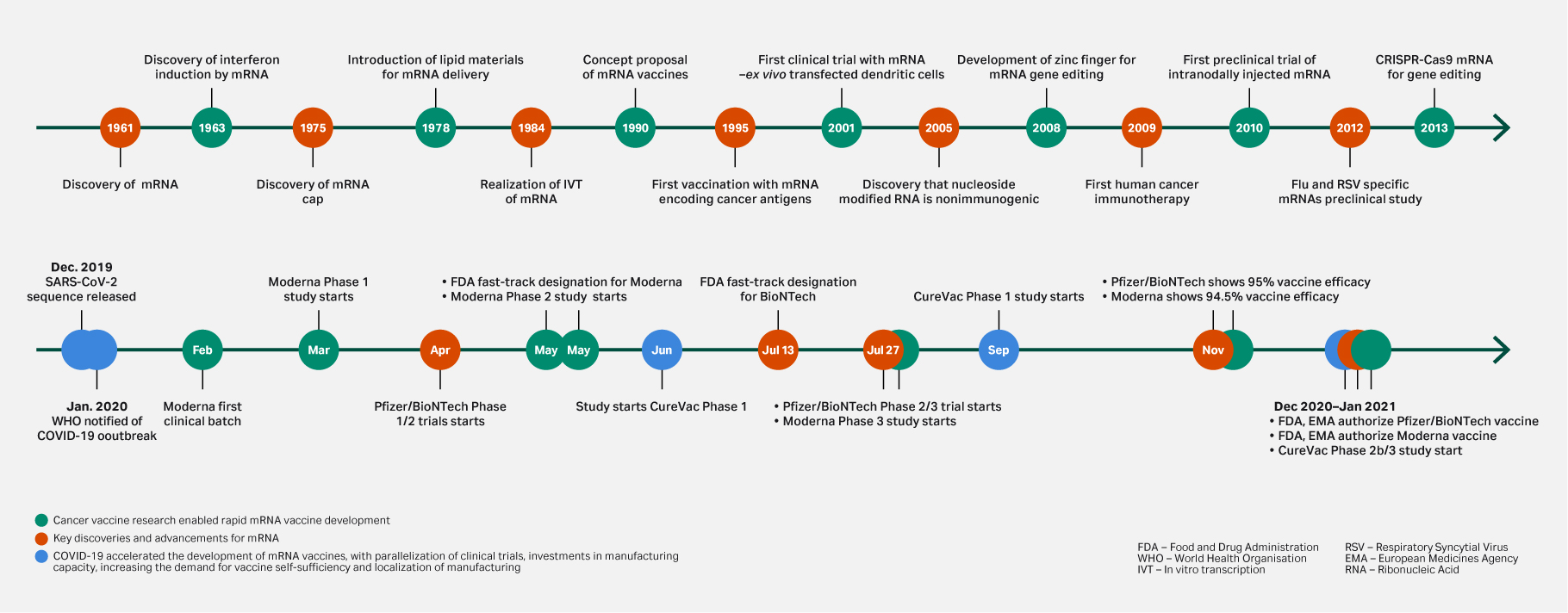
The rapid development, commercialization, and widespread distribution of COVID-19 mRNA vaccines has helped to accelerate the development of nucleic acid-based therapeutics. As well as proving their potential for rapid development and manufacturability at all scales, the COVID-19 vaccines also established a baseline with a positive safety profile. Since the success of these vaccines, mRNA therapeutics have gained traction, with increased funding and research opening doors to “new” treatment pathways. It is easy to overlook that their history is founded in cancer vaccine research and the early discoveries dedicated to this (Fig 1).
Fig 1. Key discoveries and advancements for mRNA. Cancer vaccine research enabled rapid mRNA vaccine development.
Beyond infectious diseases, there is a continued investment in this modality in the areas of immuno-oncology and autoimmune diseases, with nucleic acid-based therapeutics accounting for just over a quarter of the cancer vaccines in current clinical development.
The rise of mRNA-based therapeutics following the success of COVID-19 vaccines has had its fair share of publicity, but we shouldn’t forget that nucleic acid-based therapeutics also includes DNA-based therapeutics in the form of oligonucleotides. Oligonucleotide therapeutics are well established, with the first clinical approval occurring in 1998. But the space has evolved rapidly to treat a wide range of disease areas, with oncology and genetic disorders heavily featured.
Nucleic acid technologies have clearly demonstrated potential to treat diseases by targeting their genetic blueprint in vivo (1). They have proved that they are a promising modality for future healthcare. However, they may also have a positive impact on our planet’s future with a potential to reduce the environmental impact of drug manufacturing.
mRNA therapeutics
Compared with traditional vaccine platforms, such as live-attenuated or viral-vectored vaccines, mRNA vaccines can be manufactured faster as they do not rely on cell-based components in production. This reduces the number of optimization processes needed. The timeline for manufacturing and release of a clinical-grade vaccine will always be platform dependent, but mRNA vaccines offer the potential to be completed in as little as five weeks (2). In comparison, viral vector systems can take around six to 36 months (3). This increased speed equals an attractive reduction in energy costs, chemicals, consumables, and waste. Budzinski et al (4) demonstrated that 80% of environmental impact for biopharmaceutical manufacturing comes from electricity consumption within the manufacturing facility. With an optimistic timeline, even the quickest alternative therapies would take 24 weeks longer to manufacture than mRNA therapies.
When compared to other cell-based vaccine manufacturing processes, the relative simplicity of the mRNA manufacturing process, can provide unexpected environmental benefits in the form of smaller manufacturing plants. Platforms designed for mRNA manufacturing enable flexibility and versatility to be built into their design. Platforms for mRNA-based drug substances allow for quick adaptation to produce any RNA sequence from the same process with just a change in the DNA template. These manufacturing platforms enable quick pivots between different therapeutics and for variations in capacity needs. In contrast, other manufacturing platforms may require entirely new sets of manufacturing equipment or even new facilities to accommodate these changes.
Environmental impact of mRNA manufacturing
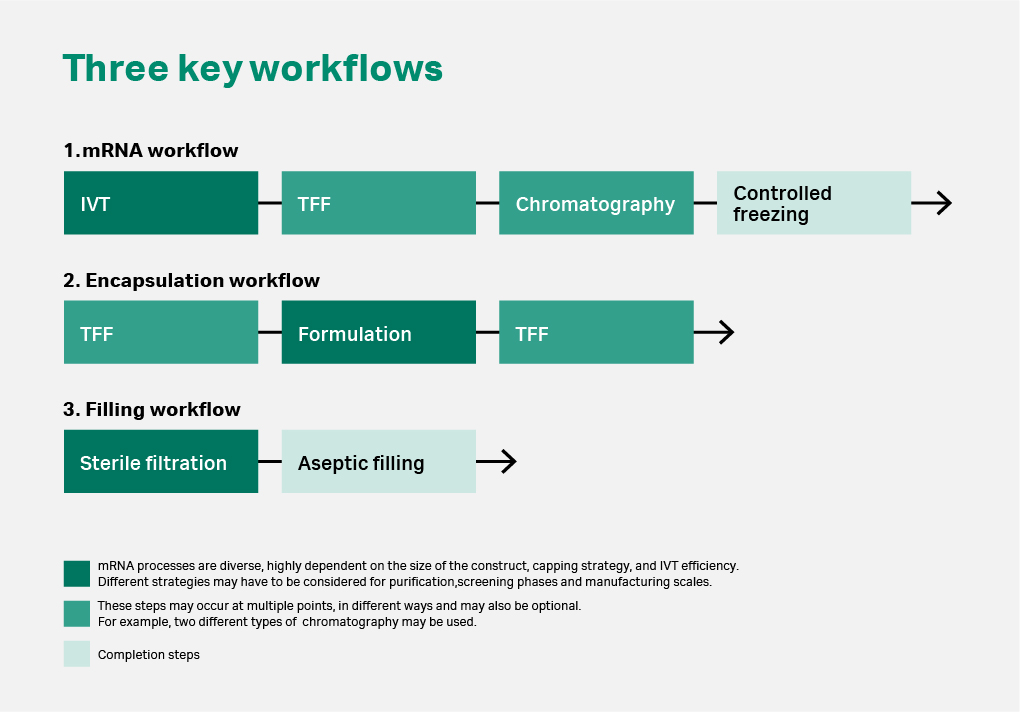
Comprehensive analysis of the mRNA process isn’t possible due to the complexity and diversity of mRNA therapeutics. There is no single standard process adopted by all manufacturers to use for direct comparison at this time. However, we can still review the environmental impact of a typical mRNA manufacturing process (Fig 2).
Fig 2. Key workflows for mRNA manufacturing processes. Manufacturing of mRNA usually starts with in vitro transcription (IVT), a biochemical process to synthesize mRNA from a DNA template. This process will usually occur in a bioreactor or a controlled mixing platform. Following this step, a series of separation and purification steps occur in the form of tangential flow filtration (TFF) and chromatography. For mRNA-LNP therapeutics, the mRNA will then be encapsulated within liquid nanoparticles (LNPs) to deliver the final injection drug product.
In their paper, Gao and Benyahia (5) mapped out the life cycle assessment of the mRNA manufacturing process steps to capture the environmental footprint of an mRNA plant. They divided this into six main stages:
- Upstream processing
- First filtration (TFF1)
- Chromatography
- Second filtration (TFF2)
- Encapsulation
- Final filtration (TFF3)
They determined that TFF2 exhibits the largest contribution in all impact categories due to the large amount of solvent used, waste generated, and quantities of sodium citrate added as a buffer. The second largest contribution in terms of environmental impact was TFF3. Goa and Benyahia proposed recycling strategies that could help mRNA manufacturers reduce the environmental impact in the process further.
Greenhouse gas emissions, single-use technology, and transportation
In a paper by Kurzweli et al (6), the ecological footprint of COVID-19 mRNA vaccines in terms of greenhouse gas emissions was assessed as a whole, from development to transport and supply to clinics. Although it is not possible to accurately calculate the impact due to the complexity and diversity mentioned earlier, the transport of vaccines was shown to contribute towards 99% of the total carbon footprint in a full lifecycle assessment. This transport impact would be present for all vaccines regardless of manufacturing process. They use the example of BioNTech (7) and showed that over 50 000 steps are required to produce an mRNA vaccine dose, with negative environmental impacts seen in dry ice, freezers, the use and disposal of polymers and glass, and emissions from transport, which are addressed below. However, Mahony et al (8) stated that “10% reduction in disease levels could reduce greenhouse gas emissions equivalent to 800 M tons of carbon dioxide annually,” with mRNA vaccination technologies cited as having the capacity to deliver this sustainable development goal (SDG) (9).
Manufacturing mRNA makes use of single-use technology, which reduces or eliminates the need for extensive cleaning and steam sterilization between batches. Indeed, it is the combination of moderate facility footprint requirement for mRNA manufacturing and the diversity of single-use components that enables rapid scale-up and scale-out (10). However, single-use technologies can also have negative environmental impacts due to the use and disposal of consumable materials. There have been several studies comparing the environmental impacts of single-use manufacturing technologies in comparison to traditional multi-use process equipment such as stainless-steel fermenters. Pietrzykowski et al (11) concluded that adopting single-use technology has less environmental impactful than traditional equipment. This lower impact was due to the decreased energy costs of steam sterilization for components, process water, and water for injection, maintenance, and optimizations runs.
Many mRNA therapeutics produced to date require low-temperature, cold-chain storage. However, emissions generated from last-mile delivery accounts for nearly 19 times the emissions generated from all other impacts. Ensuring that ultra-cooling units are of a newer, energy-efficient design helps reduce the impact of storing at low temperatures. Research continues into the stability of lyophilized mRNA-LNP vaccines to align with green transport goals (12).
Benefits of hub-and-spoke model for mRNA therapeutics distribution
Manufacturing of mRNA can lend itself to a hub-and-spoke model as promoted by the World Health Organization (WHO) (13) to low- and middle-income countries. This model enables a hub facility to produce mRNA vaccines through a center of excellence working with a network of technology recipients (spokes). Adopting such a model can mean that manufacturing facilities can stay smaller and react to capacity demand. It also serves to reduce the amount of transport needed such as overseas shipping.
Distributed manufacturing brings small-scale manufacturing to many locations as opposed to the centralized, mass-production paradigm. An example of this biofoundries, which separate design from manufacturing. Highlighted in Kitney et al (14), “biofoundries incorporate high levels of automation that implement complex workflows in order to increase the reliability and reproducibility of biotechnology.” Biofoundries work by communicating via digital data. For example, a vaccine sequence could be designed in one facility and then sent digitally to a manufacturing facility. Next generation vaccines such as those based on mRNA are particularly suited to this model due to their ability to have smaller facility designs. Designing vaccines in biofoundries with distributed manufacturing in small scales is possible with mRNA and provides environmental impact positives in the form of reduced transport and cold-chain transfer and smaller manufacturing footprints.
For the next generation of RNA vaccines, we are now seeing variation with RNA forms such as self-amplifying RNA (saRNA). A benefit of saRNA-based vaccines is that the amount required per dose is much less and therefore will require less materials to produce. Kis et al (15) theorized that small-scale processes enabled by lower dose saRNA vaccines will require a lower number of resources to set up and operate.
Oligonucleotide therapeutics
Oligonucleotide therapies are more established than mRNA, with more therapies in the later stages. Oligonucleotides can bind to complementary DNA or RNA sequences, so they can be used to modify and/or modulate gene expression by inhibition, activation, induced degradation, or splice-switching, or they can fold on themselves to form three-dimensional structures called aptamers, which can bind to target disease-causing proteins. The manufacture of oligonucleotides consists of four stages:
- Synthesis
- Cleavage and deprotection
- Purification
- Isolation or desalting
These processes are usually flexible and designed to fit the need of scale-up for later stage clinical applications as well as commercial needs. Traditional oligonucleotide processes have had a large environmental impact in terms of high-volume acetonitrile solvent, large volumes of waste, chemical hazards, and energy. With the increased interest in oligonucleotide therapeutics, manufacturers and researchers are working to identify where significant savings can be made in terms of environmental resources.
Environmental impact of oligonucleotide manufacturing
Like mRNA therapeutics, oligonucleotide therapeutics are cell-free processes and thus benefit from the same reduction in optimization needed and length of time to manufacture, which enables positive environmental impact in the form of reduced electricity consumption. Unlike mRNA therapeutics, oligonucleotide manufacturing is already well established with standard processes in place. The main challenges faced by oligonucleotide manufacturers are scaling up processes, which is usually accomplished by modernizing facilities, optimizing machinery, securing supply chain for large volumes of solvents and chemicals, optimizing facility design to handle such volumes, training and retaining employees, and making synthetic processes more efficient.
In comparison to mRNA, the oligo synthesis process is a chemically driven process, which has had a negative environmental impact in the past. However, the increased interest in this sector has driven the implementation of a greener mindset, enabling sustainability to be built into the process. Early efforts focused on reducing and optimizing washing volumes in each process step, hence minimizing waste, and reducing the cost. Currently there is a movement towards ion exchange chromatography (IEX) or even hydrophobic interaction chromatography (HIC) to replace the traditional RPC. These changes can lead to a reduction in hazardous waste disposal.
The synthesis stage is responsible for half of the materials used in the whole process and manufacturers can look at reducing wash steps (16). Purification is the other major material usage step, where manufacturers can look at HIC and IEX as an alternative for RPC, which has a high environmental cost in the form of hazardous waste. Sanghvi et al (17) detailed how manufacturers can achieve greener chemistry in these steps. “Longer term goals could involve improving compound design or investigating alternative methods of synthesis and purification.”
Conclusion
The cell-free processes of nucleic acid-based therapeutics enable rapid manufacturing with reduced optimization needs thus reducing the overall energy consumption and material usage within these processes. Oligonucleotide therapeutics and mRNA therapeutics are benefiting from increased investment in the field, which is helping to drive efficiency into processes.
Regardless of whether environmental impact is assessed purely on greenhouse gases or the lifecycle impact of manufacturing facilities, these therapeutics offer environmental savings in the form of enabling reduced transport via biofoundries, enabling reduced electricity consumption via reduced manufacturing times, or enabling reduced chemical waste through single-use solutions and optimized manufacturing processes. With sustainability being a key focus for manufacturers, nucleic acid-based therapeutics provide optimal potential for improving cost and efficiency in processes and reducing environmental impact in tandem.
CY41954-18Mar24-AR
- Kulkarni JA, Witzigmann D, Thomson SB, et al. The current landscape of Nucleic acid therapeutics. Nat Nanotechnol. 2021;16(6):630-643. doi:10.1038/s41565-021-00898-0
- Saville M, Cramer JP, Downham M, et al. Delivering pandemic vaccines in 100 days — what will it take? N Engl J Med. 2022;387(2). doi:10.1056/nejmp2202669
- Rosa SS, Prazeres DMF, Azevedo AM, Marques MPC. mRNA vaccines manufacturing: challenges and bottlenecks. Vaccine. 2021;39(16):2190-2200 doi:10.1016/j.vaccine.2021.03.038
- Budzinski K, Constable D, D’Aquila D, et al. Streamlined life cycle assessment of single use technologies in biopharmaceutical manufacture. N Biotechnol. 2022;68:28-36. doi:10.1016/j.nbt.2022.01.002
- Gao S, Benyahia B. Technoeconomic and life cycle assessment of an mRNA vaccine integrated manufacturing plant. Computer Aided Chemical Engineering. Published online 2023:2261-2266. doi:10.1016/b978-0-443-15274-0.50360-7
- Kurzweil P, Müller A, Wahler S. The ecological footprint of covid-19 mRNA vaccines: Estimating greenhouse gas emissions in Germany. Int J Environ Res Public Health. 2021;18(14):7425. doi:10.3390/ijerph18147425
- BioNTech Provides Update on Vaccine Production Status at Marburg Manufacturing Site, Press Release, Mainz, 26 March 2021. Available online: https://investors.biontech.de/de/news-releases/news-release-details/biontech-gibt-update-zu-status-der-impfstoffproduktion-der (accessed on 21 Jan 2024)
- Mahony TJ, Briody TE, Ommeh SC. Can the revolution in mRNA-based vaccine technologies solve the intractable health issues of current ruminant production systems? Vaccines. 2024;12(2):152. doi:10.3390/vaccines12020152
- The United Nations: 17 Goals. https://sdgs.un.org/goals Accessed Feb 2024
- Grilo AL, Schmidhalter DR. mRNA manufacturing and single‐Use Technology – A perfect liaison. Chem Ing Tech. 2022;94(12):1920-1927. doi:10.1002/cite.202200147
- Pietrzykowski M, Flanagan W, Pizzi V, Brown A, Sinclair A, Monge M. An environmental life cycle assessment comparison of single-use and conventional process technology for the production of monoclonal antibodies. J Clean Prod. 2013;41:150-162. doi:10.1016/j.jclepro.2012.09.048
- Ai L, Li Y, Zhou L, et al. Lyophilized mRNA-lipid nanoparticle vaccines with long-term stability and high antigenicity against SARS-COV-2. Cell Discov. 2023;9(1). doi:10.1038/s41421-022-00517-9
- World Health Organization: The mRNA vaccine technology transfer hub. https://www.who.int/initiatives/the-mrna-vaccine-technology-transfer-hub (Accessed Jan 2024)
- Kitney RI, Bell J, Philp J. Build a sustainable vaccines industry with synthetic biology. Trends Biotechnol. 2021;39(9):866-874. doi:10.1016/j.tibtech.2020.12.006
- Kis Z, Kontoravdi C, Shattock R, Shah N. Resources, production scales and time required for producing RNA vaccines for the global pandemic demand. Vaccines. 2020;9(1):3. doi:10.3390/vaccines9010003
- Andrews BI, Antia FD, Brueggemeier SB, et al. Sustainability challenges and opportunities in oligonucleotide manufacturing. J Org Chem. 2020;86(1):49-61. doi:10.1021/acs.joc.0c02291
- Sanghvi YS, Ravikumar VT, Scozzari AN, Cole DL. Applications of green chemistry in the manufacture of oligonucleotide drugs. Pure Appl Chem. 2001;73(1):175-180. doi:10.1351/pac200173010175