Why security of supply matters
The reliable supply of biological based medicinal therapies is highly dependent on a robust, well planned, and risk-managed supply chain. Therefore, business continuity management has become a critical discipline of suppliers in the bioprocess industry. Their goal is to deliver products and services that incorporate risk-mitigating supply chain controls and have a high degree of supply assurance and resilience to supply disruptions.
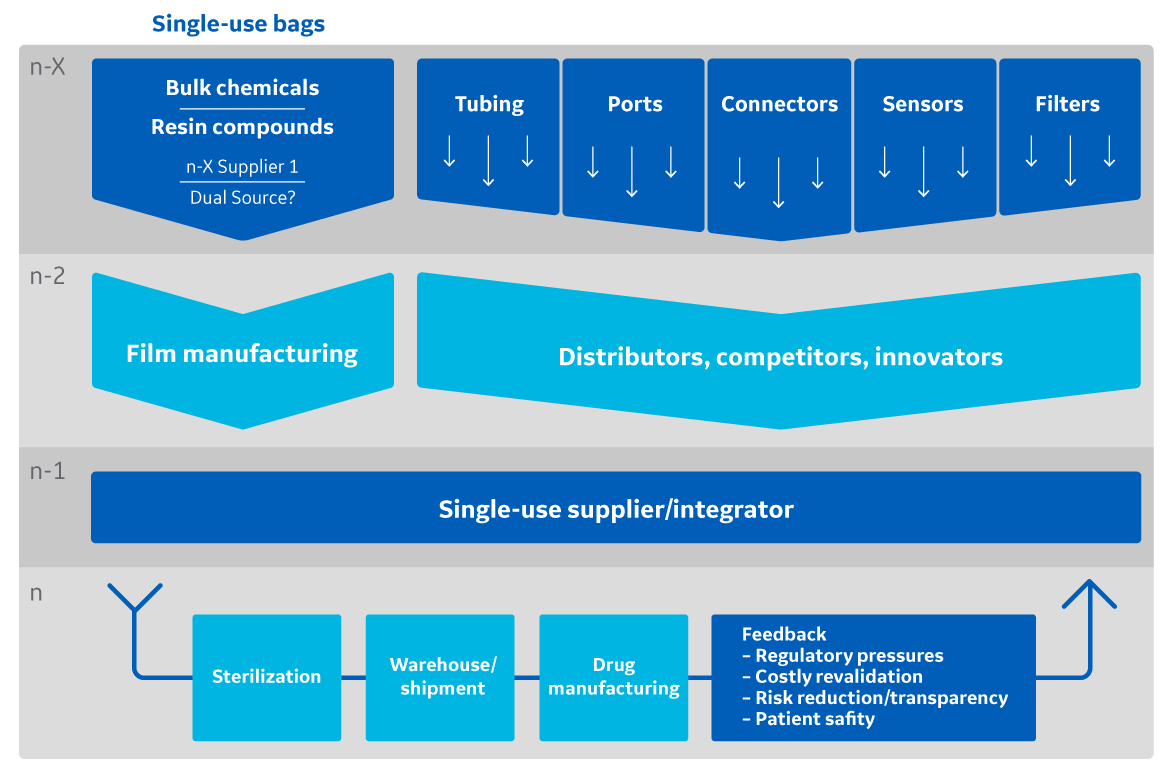
Single-use technologies continue to see rapid growth and adoption in clinical and commercial biomanufacturing processes where speed and flexibility are desired. Along with these advantages, the shift toward single-use processing creates a paradigm shift for these manufacturers. Their ability to maintain supply becomes increasingly reliant on external suppliers as equipment shifts from batch-to-batch maintained stainless steel systems to disposable ones, which must be procured, delivered, released, and installed for each batch startup. Single-use bags for bioreactors, mixers, culture media, and process fluids are all manufactured from polymer-based films that make up the vast majority of the single-use system’s product contact surface area. In this context, the supply assurance of bioprocess systems and the therapies they produce are dependent on the secure supply of bioprocess film. These realities explain why biomanufacturers rely on supply control and assurance throughout the single-use bag supply chain—from film raw materials to manufacturing into film and, eventually, to the delivery of finished bioprocess systems.
Industry learnings for single-use product development
In the early life cycle of single-use product development, systems were constructed primarily from multilayer films designed for food and medical applications. Using these films in bioprocessing led to the discovery that materials in the film can impact cell cultures. For example, growth inhibition was observed for sensitive Chinese hamster ovary (CHO) cell lines. After a lengthy investigation, the root cause was identified as an interaction with a film leachate, more specifically, a degradant from a common antioxidant used as a processing aid to manufacture the film. The biomanufacturer and supplier community discovered during the investigation that there were few controls of the quality attributes of multilayer films critical to bioprocess applications.
Underscoring these challenges was that single-use products for bioprocessing represent a small portion of the global demand for plastic resins. Although this limited financial leverage over plastic resin suppliers, the industry sought solutions to control and minimize supply risk in bioprocessing.
Based on the learnings from first-generation materials, supply chain stability became a focus for industry working groups and consortia. In response to this realization and industry need, the Biophorum Operations Group (BPOG) released a guidance document in the form of white paper titled Supply Chain Mapping: A Best Practice for the Biopharm Industry. The connection to risk management is simply and succinctly stated in a section on security of supply.1 It states, “The dynamics of a global economy for materials and the need to ensure specific regulatory and legislative compliance makes the security of supply a major challenge. Drug companies and supply partners need to understand the risks involved and undertake due diligence to minimize their impact on the supply chain.”
Any step along this broad and deep supply chain poses a challenge to ensuring that bioprocess films consistently meet their predetermined quality attributes required for biomanufacturing. Therefore, increasing supplier awareness, integrating quality controls, and securing material contracts have become critical elements of new bioprocess film development projects.
Fig 1. Schematic representing the complexity and depth of a supply chain for single-use bags.
Single-use film built for bioprocessing
For the next generation of single-use products, biomanufacturers asked for improvements in supply chain control, consistency, and business continuity. These requests, along with the need to enhance performance across applications, were the foundation for building a platform film specifically for bioprocessing.
Cytiva chose Sealed Air Corporation as its strategic partner for the development and construction of a new platform film, Fortem™.2 With a 30-year history serving the medical industry, Sealed Air’s knowledge, size, and scope were essential for navigating technical development nuances and setting contracts with resin suppliers to meet performance and supply requirements. Three aspects were considered when developing and maintaining Cytiva's single-use platform film, Fortem and its security of supply program, which align with Cytiva's overall security of supply framework for bioprocessing:
1. Supply chain control
Today, thanks to biopharma industry consortia and outreach, plastic resin suppliers are increasingly aware of their role in serving the biomanufacturing industry. However, it is still necessary to carefully implement robust supply chain controls.
Final product testing of films (e.g., cell culture compatibility) is one way to verify quality and supply integrity and detect nonconformance. Yet, this approach does little to improve quality and supply assurance. Instead, it simply verifies the result and outcome of the entire value chain. Starting with a firm understanding of film aspects that impact biomanufacturing, it is possible to implement effective controls governing the critical to quality (CTQ) aspects throughout the supply chain.
In a quality by design (QbD) approach, controls are established by setting contractual specifications, CTQ aspects, and critical process parameters with material sub-suppliers. However, it is difficult to implement the output of QbD programs as specialized controls across global supply chains, which are subject to competing market forces.
By leveraging Sealed Air’s industrial strength in food and packaging films, Cytiva gained access to established, strategic supplier relationships. This framework allowed Cytiva to capitalize on commodities and secure conditions for specialized needs. Sealed Air helped us to influence large, highly capable commodity material players to adapt to some of the niche needs of the biopharma industry. Gaining that level of supply partnership with securely established players is a benefit to the overall health of the supply chain.
2. Business continuity
Security of supply was top of mind during the development of Fortem. Business continuity program leaders from Cytiva and Sealed Air discussed strategies for protecting against interruptions—both within the supply chain and also at Sealed Air’s or Cytiva's manufacturing facilities.
Together, the companies obtained contractual obligations from sub-suppliers of resins, additives, and compounded resins who agreed to a change notification period of two years prior notice, with up to two years of last-buy material in the event of an obsolescence notification. As a result, Cytiva customers have four years to implement any material changes. That allows Cytiva customers a good line of sight to change. It also allows Cytiva and Sealed Air time to work together to understand what the change is and how to minimize any adverse impact of it. If a change is necessary, biomanufacturers will receive notification and sufficient information to evaluate the impact on their products and processes as far in advance as possible.
Because of the long successful history of Cytiva biopharma’s chromatography resin being designed into many commercial drug processes, Cytiva has a strong vantage point to understand the critical supplier obligations. As such, it has made investments to supply assurance and business resiliency in case something goes wrong. For example, Cytiva supports security of supply of its chromatography resins through site business continuity operations and safety stock philosophies.
In the unlikely event of a plant loss at Sealed Air’s site in Duncan, SC, where Fortem is made, Sealed Air has contingency plans to transfer Cytiva's film manufacturing process to an alternate site with the same extrusion equipment. The quantity of finished film safety stock maintained by Cytiva was established based on the duration of the second site contingency plan. These risk mitigating actions keep bioprocess bags supplied even if film production was interrupted. Trust and putting the patient first are critical pre-requisites to sharing such business-critical information, such as crisis contingency plans and safety stock strategies.
As is true for Cytiva's site in Uppsala, Sweden, where chromatography resins are manufactured, the single-use manufacturing site in Westborough, MA, is accredited to the ISO 22301:2012 standard.3 This external certification demonstrates the ability of a facility to prevent or minimize the effects of a disruption through dedicated crisis response plans and management teams. It carries additional requirements for continued site improvement. Bringing critical manufacturing sites into that accreditation is part of Cytiva's overall security of supply strategy for bioprocessing.4
3. Communication
The biopharmaceutical industry understands that supply chain control, as described above, is best achieved when there is transparency between companies. Information sharing has been limited in the past for many reasons, such as intellectual property confidentiality. While there are no existing standards for information sharing, the industry is developing best practices through consortiums like BPOG. As such, it is up to bioprocess consumable suppliers to listen to the needs of biomanufacturers and establish their own policies. The need for transparency by biomanufacturers in regard to raw material characterization, qualification, and supply chain is also expressed in the BPOG supply chain mapping guidelines, which state, “The difficulties, and perhaps sensitivities, that are historically prevalent in parts of the industry can be overcome through more widespread use and acceptance of the need for transparency and use of a more standard approach at, and through, the various supply chain tiers.”1
Both Cytiva and Sealed Air have shared details relevant to these biomanufacturers’ needs. In addition to discussing the film formulation, the companies shared information about supply chains and quality controls, while Sealed Air provided transparency to its suppliers and purchasing specifications.
This openness also extended beyond Sealed Air’s own operations, deep into its supply chain. When Cytiva wanted the ability to share the complete film formulation down to the chemical abstract services (CAS) number with its customers, Sealed Air understood intellectual property could not be exposed. This information is important to Cytiva's subset of customers, as the information is used to ensure public safety.
This information, along with the full film characterization and validation efforts, is available to Cytiva biopharma customers through our regulatory support files portal.5
Summary
Developing a film specifically for bioprocessing has allowed Cytiva to focus squarely on biomanufacturers’ needs. Building from the ground up assured a strong supply chain from the beginning. In addition, the relationships that Cytiva's partner, Sealed Air, has with its plastic resin suppliers enabled tight quality and supply controls throughout the supply chain. As a result, adopters of Fortem benefit from up to four years of notice if any component in the film will change. In addition, to help protect customers from supply disruptions, Cytiva's Westborough site has received ISO accreditation for business continuity.
Ultimately, communication is the most critical ingredient for the end-to-end supply chain. Transparent and timely communications between suppliers, sub-suppliers, and biomanufacturers will ensure that a continuous supply of life-improving therapies are developed and produced using single-use systems.
About Cytiva's security of supply framework
The strategies for Fortem film security of supply are consistent with Cytiva's overall framework for materials used in approved processes. Cytiva takes a risk-based, comprehensive, and cost-efficient approach to security of supply. Three elements encompass Cytiva's holistic approach:
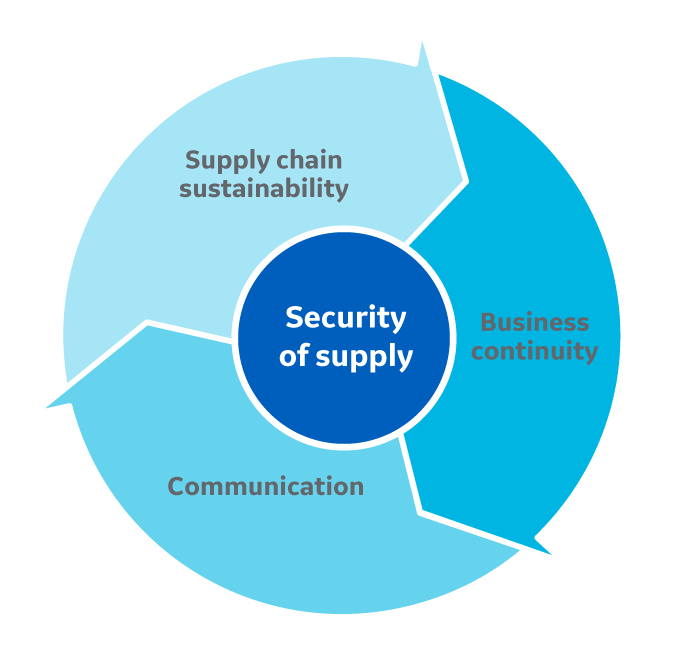
Fig 2. Elements of Cytiva's security of supply framework for biomanufacturers.
- Supply chain sustainability means proactive management of supply, quality, and capacity. Supply chain control, supply consistency, and risk mitigation are important aspects. Cytiva's quality-by-design (QbD) approach to product and manufacturing process design ensures that biomanufacturing processes perform consistently. Investments in continuous improvement and capacity expansion give Cytiva the ability to match an organization’s manufacturing growth.
- Business continuity is another term for emergency preparedness. Dual-site manufacturing, safety stock, and long-term agreements are strategies for business continuity. Because product supply chains and manufacturing processes vary, a tailored approach is most effective at preventing or minimizing the impact of any event, great or small.
- Communication is about sharing and increasing knowledge. Transparency, regulatory support, and collaboration are reasons to communicate. A foundation of transparency and trust enables Cytiva to effectively support biomanufacturers’ needs. Collaboration leads to better results through increased product innovation and involvement up and down the supply chain.
- Maclea, Rod, et. al. (April 2018). Biophorum Operations Group. Supply Chain Mapping: A Best Practice for the Biophorum Industry? (pg. 7; section 4.3)
- Cytiva Newsroom, Cytiva and Sealed Air enter an exclusive agreement to deliver film for single-use bioprocess systems. Retrieved from https://www.genewsroom.com/press-releases/ge-healthcare-and-sealed-air-enter-exclusive-agreement-deliver-film-single-use
- International Organization for Standardization, ISO 22301:2012, Societal Security, Business Continuity Management Systems, Requirements. Retrieved from https://www.iso.org/standard/50038.html
- Cytiva, Security of Supply. Retrieved from https://www.gelifesciences.com/solutions/bioprocessing/products-and-solutions/security-of-supply
- Cytiva, Regulatory Support, Retrieved from https://www.gelifesciences.com/en/us/support/quality/regulatory-support