By Lawrence Okorafor - Scientist II, Morven McAlister - Senior Director and Nigel Jackson - Principal Engineer, R&D
Virus filtration is performed to ensure viral safety in biotherapeutic manufacturing processes. By applying new industry knowledge, we have successfully demonstrated how to overcome many of the challenges in the industry today: the impact of low pressure on virus breakthrough, upstream virus filtration for ATMPs (advanced medicinal therapeutic products), validation challenges posed by adsorptive prefilters, and lower pressure and longer processing times associated with continuous virus filtration. The results of the study provide you with valuable insights to make informed decisions for virus filtration as incorporated in both PDA TR - 41 (2022) (Parental Drug Association Technical Report – 41) and the ICH-Q5A (International Council for Harmonization) update.
Introduction
Virus filtration is a robust mechanism to ensure virus safety of biotherapeutic manufacturing processes. A virus filtration step must be validated under simulated worst-case processing conditions to meet regulatory expectations, hence creating a suitable design of experiments (DoE) is critical. This presents several challenges including virus breakthrough resulting from low pressure or depressurization (stop and start), implementation of prefilters, complexities of intensified or fully continuous processes, and therapeutic products from new manufacturing modalities like advanced therapy medicinal products (ATMPs). The difficulty in applying traditional viral clearance strategies directly to ATMPs confirms that novel virus risk reduction approaches such as removal of potential viral contamination from raw materials are of increasing priority.
The updates to PDA TR - 41 (2022)1 and ICH Q5A(R2)2 address these new challenges and requirements for our increased knowledge regarding worst-case parameters for virus filtration. Our study demonstrates some of the filtration methods we have developed to overcome these challenges, contributing to the overall viral safety assurance of biologics.
Background
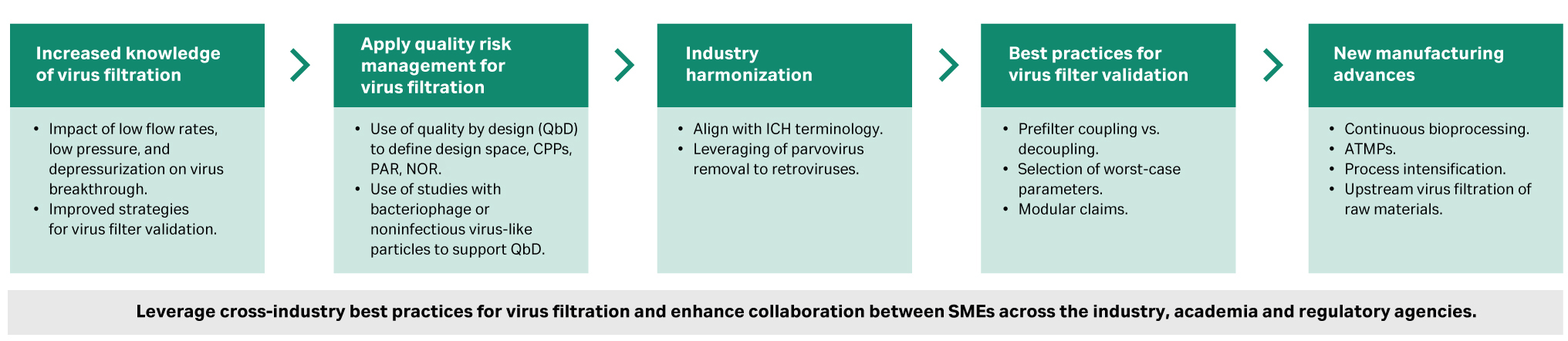
The updates to PDA TR - 41 and ICH Q5A (R2) ensure that various virus filtration challenges encountered since the original issue of these documents in terms of virus breakthrough, low pressure, or pressure interruption as well as validation issues have been addressed. The updates aim to leverage cross-industry best practices for virus filtration and validation and ensure alignment with ICH terminology (Fig 1).
Fig 1. Key areas highlighted by the recent update in PDA TR -41. (CPPs = Critical Process Parameters, PAR = Proven Acceptable Range, NOR = Normal Operating Range)
We have performed a series of experiments to overcome the filtration challenges posed by four of the key areas highlighted in the revised PDA TR - 41 which include:
- Impact of low pressure and stop/start on virus breakthrough.
- Upstream virus filtration for ATMPs (gene therapy).
- Validation challenges due to an adsorptive prefilter.
- Continuous virus filtration.
Results and discussion
Pressure interruptions
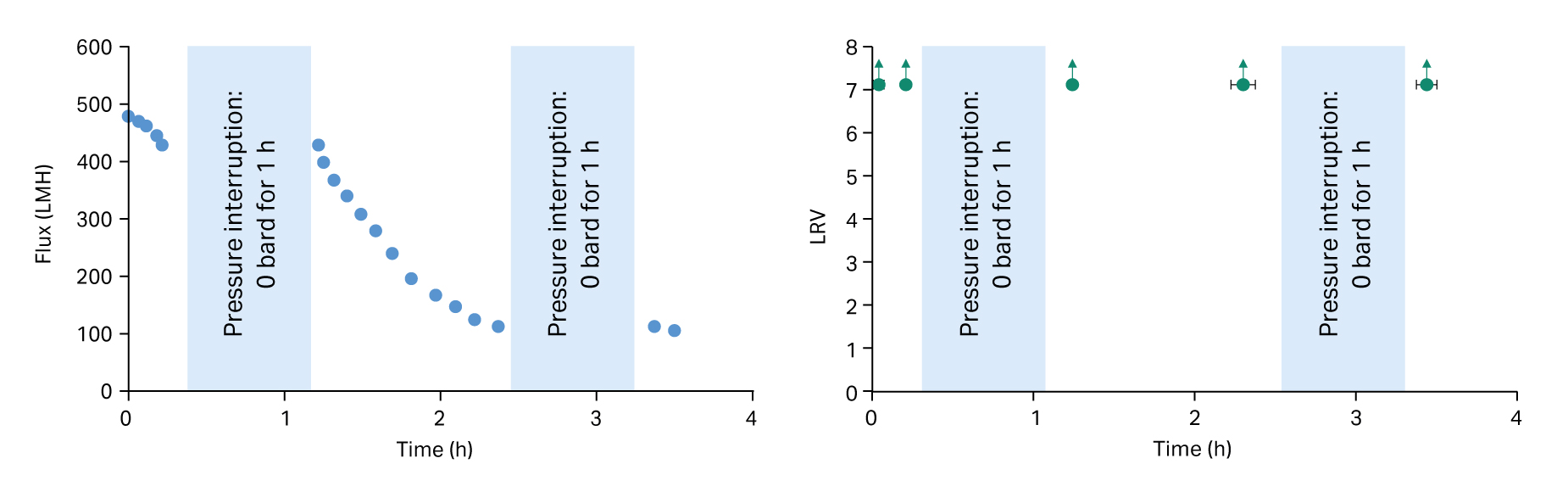
Due to the known risk to retention performance, current regulatory expectation is that virus filtration validation studies include at least one depressurization during the test. Multiple interruptions may have more impact on virus retention compared to one long pressure interruption. Pressure interruptions early or late in the process may impact on viral safety. Figure 2 shows one of the many virus validation studies performed with multiple pressure interruptions early and late in the process, showing no observed difference in log reduction value (LRV) before and after each 1 h stop. This is part of a wider data set on various pressure interruption scenarios with bacteriophage that improves our prior knowledge and contributes to the implementation of QbD principles for virus filtration.
Fig 2. Pegasus™ Prime virus removal filters: Pressure interruption study (2 × 1 h pauses). Immunoglobulin (IgG) in phosphate-buffered saline (PBS) spiked with bacteriophage PP7 at 2.1 bard (30 psid). Data are the mean values from triplicate tests. LRV x-axis error bars represent the size of the aliquots taken for the data point shown.
Upstream virus filtration for ATMPs
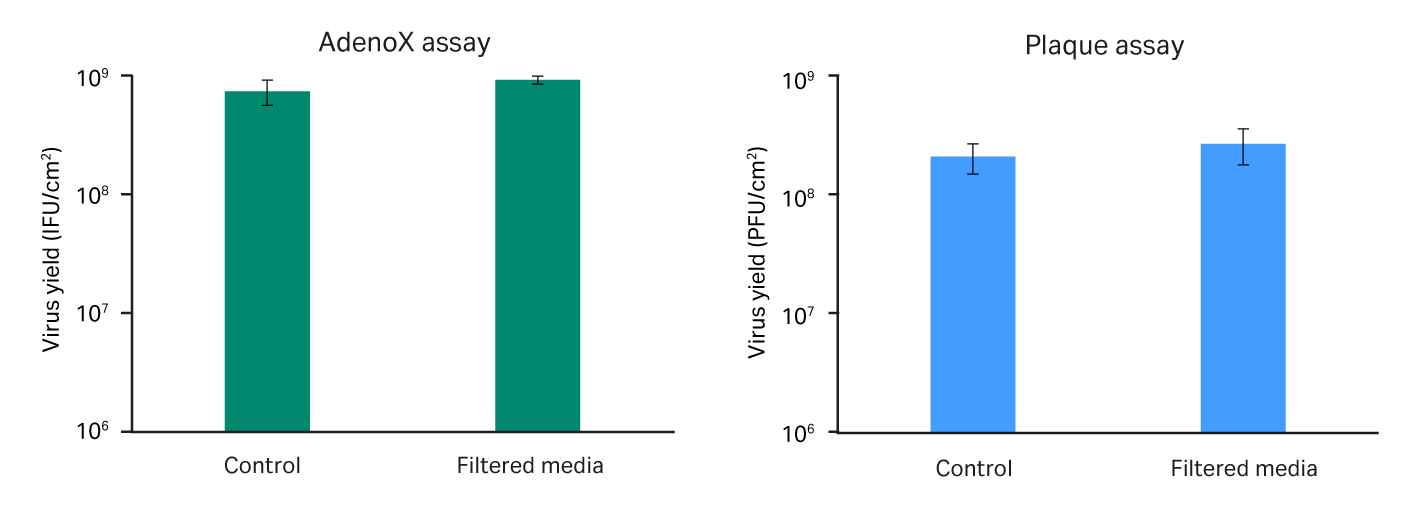
In cell and gene therapy manufacturing, there are fewer options to remove or inactivate viruses downstream because such measures could damage or remove the product itself. You should therefore focus on approaches such as upstream filtration to maximize risk reduction. There is extensive data on high viral safety of products such as Pegasus Prime virus removal filters in downstream processing. The risk in this process step is the potential impact of filtration on cell culture performance and this is where new performance verification methods are required (Fig 3)
Fig 3. Characterization of virus-filtered media (Dulbecco’s Modified Eagle’s Medium [DMEM] for HEK293, VP-serum-free medium [SFM] for Vero cells) on virus titers. Vero cells are used for making adenovirus (AV) vaccines and HEK293 for making adeno-associated virus (AAV) gene-therapy vectors.
Our results show that:
- Both control and filtered culture media support high cell viabilities.
- Filtered media has no loss in critical factors that are required for cell growth.
- Vero and HEK293 cells grew exponentially in both control and filtered cell culture media in 3 to 5 d.
- There is negligible difference in cell confluence, harvest cell density, or cell doubling time between control and filtered cell culture media.
- HEK293 and Vero cell morphology were healthy without abnormality, as evident on the day of harvest
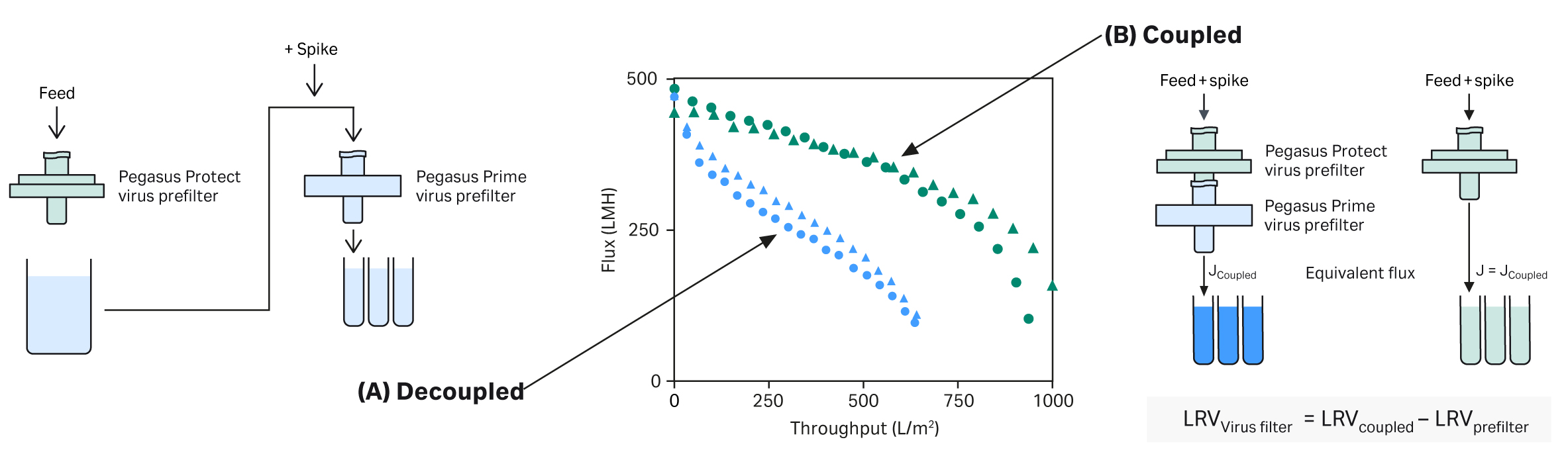
Coupling/decoupling studies
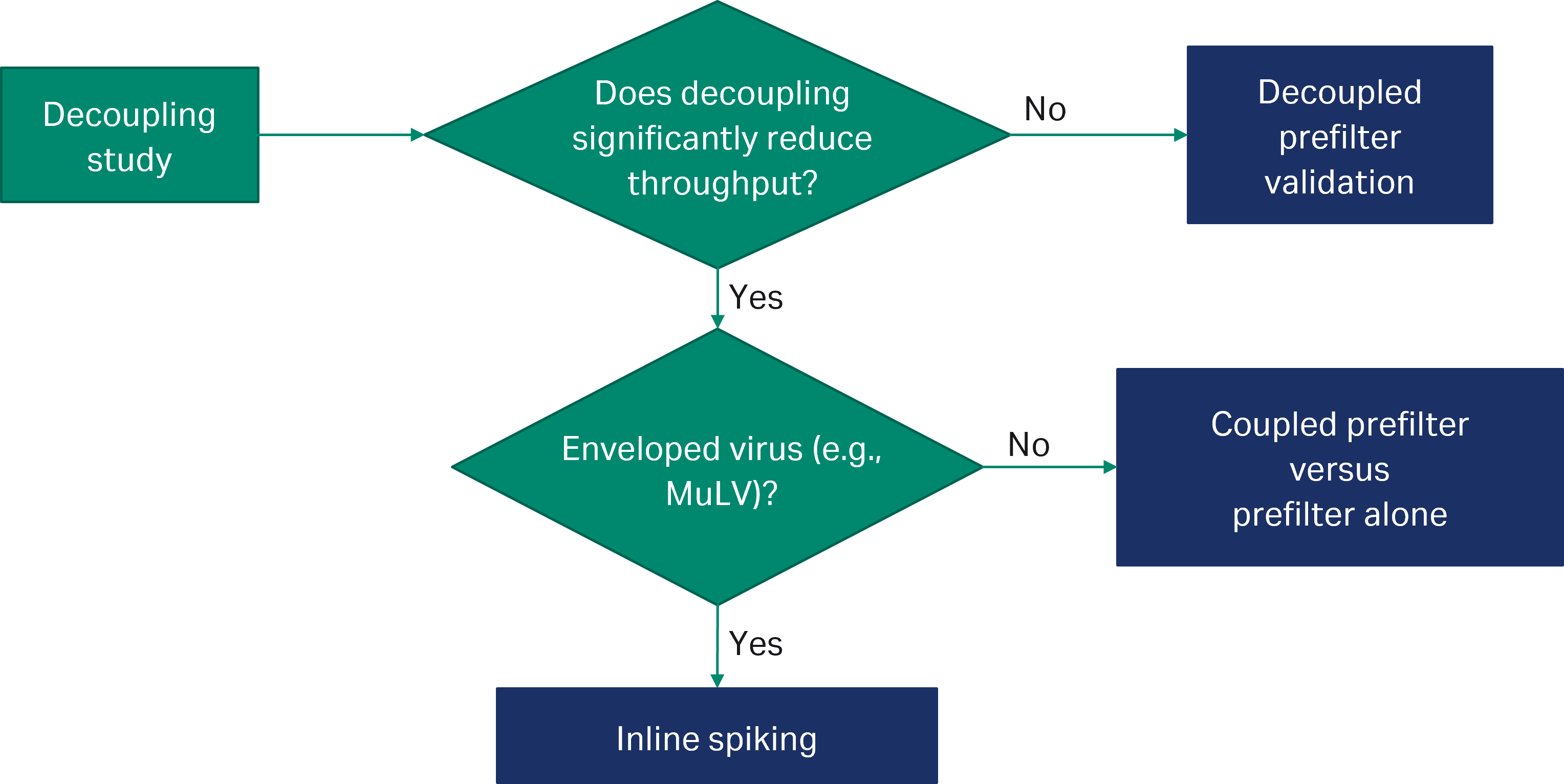
Use of adsorptive prefilters pose validation challenges for virus filtration. Aggregates removed during adsorptive prefiltration can reform over time, when the pooled prefilter filtrate is spiked and challenged to the virus filter. Aggregates lead to a reduction in throughput, a less effective process scale model and an inability to validate the target process volume per filter. Our decision tree in Figure 4 provides guidance for choosing the most appropriate test method that ensures the LRV across the device alone is determined.
Fig 4. Decision tree for virus filter validation using the Pegasus Protect prefilters. This guidance may be subject to change, and you should always contact your chosen virus validation laboratory and the regulatory agencies for confirmation.
We carried out a study to demonstrate decoupled and coupled virus validation procedures. The mAb test solution used shows lower throughput performance when tested with the prefilter decoupled compared to the coupled set up (Fig 5 and Table 1). Our data demonstrates that selecting the correct spiking method can allow for the validation of a greater process throughput.
Fig 5. (A) Set-up for validation with a decoupled prefilter, (B) Set-up for coupled prefilter vs prefilter validation.
Table 1. Maximum potential claim from duplicate clearance studies for Pegasus Prime filter. No significant virus reduction from the Pegasus Protect prefilter
| Spiking method | Volumetric throughput of mAb (L/m2) | Mass throughput of mAb (kg/m) | LRV of minute virus of mice |
| Decoupled | 636 | 5.1 | ≥ 5.9 ± 0.3 |
| Coupled | 937 (+47%) | 7.6 (+47%) | ≥ 6.1 ± 0.5 |
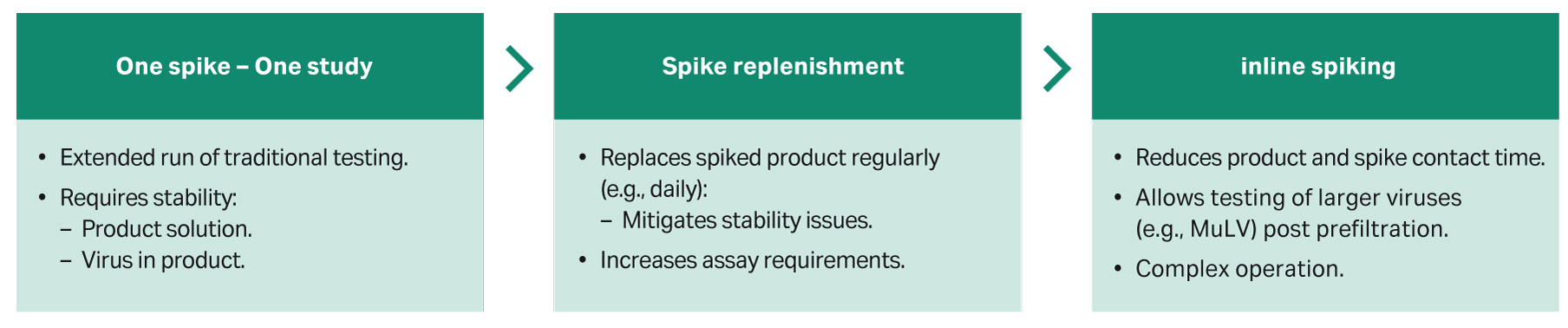
Continuous virus filtration
Continuous bioprocessing is increasingly being deployed in mAb bioprocessing due to potential advantages such as smaller facility footprints, lower investment costs, flexibility, and process economy. When virus filters are used in a continuous processing, operating conditions may be quite different to batch mode, resulting in much lower flow or pressure and longer processing times. Regulatory feedback requires that you should test the full process duration and process flux due to the risk of these factors to LRV. Figure 6 summarizes the methods developed for virus clearance testing in continuous virus filtration.
Fig 6. Virus challenge testing options for continuous virus filtration.
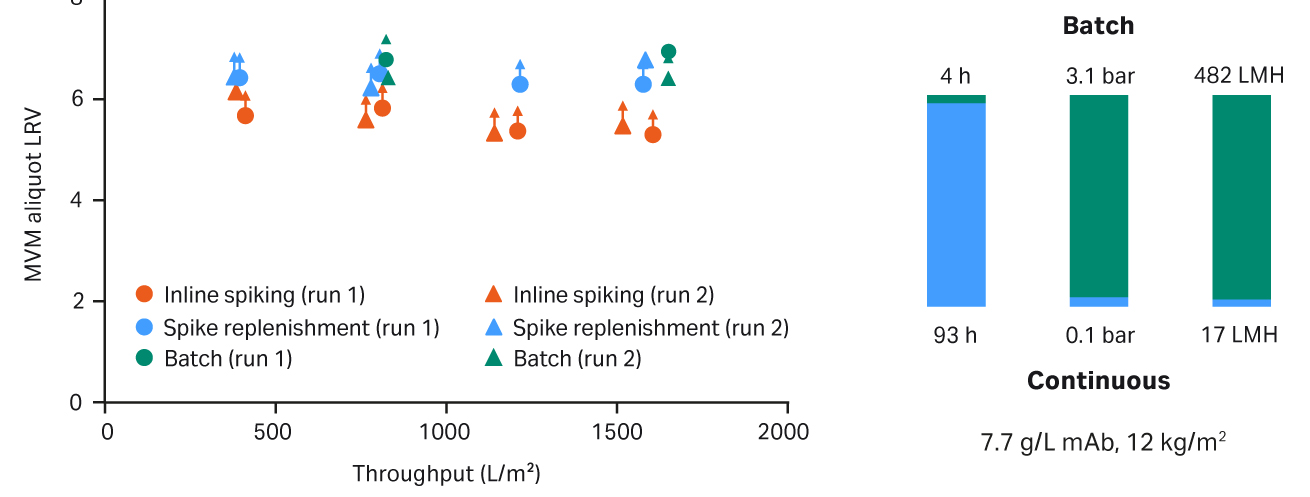
Fig 7. Pegasus Prime filter LRV using continuous virus filtration (continuous in-line spiking and continuous spike replenishment) compared to batch filtration.
By introducing techniques such as spike replenishment and improving the hold up and methods in existing techniques such as inline spiking, we have been able to demonstrate the capability to run virus challenge tests over 4 d. Throughout the test, < 0.3 log of input titer was lost and with replenishment approaches, this could be maintained indefinitely. This is exemplified in the high LRV measured across the full 4-d test to high mAb throughput, as shown in Figure 7.
Conclusions
Choosing the right filtration technique to evaluate virus clearance of your process can be quite daunting. By applying new industry knowledge to Cytiva filters, we have successfully demonstrated how to overcome many of these challenges, and provided valuable insight to make informed decisions for virus filtration in both traditional and emerging fields, as incorporated in both the PDA TR - 41 (2022) and the ICH Q5A (R2) updates.
REFERENCES
- Parenteral drug association technical report PDA TR - 41: Virus filtration; 2022.
- ICH Q5A(R2) Guideline on viral safety evaluation of biotechnology products derived from cell lines of human or animal origin; 2023.
- Validating Pegasus Prime virus removal membrane filters: How do I incorporate a prefilter in my virus clearance study. GN18.07221 19 Mar. 2018. https://cdn.cytivalifesciences.com/api/public/content/OVszlbmPvkSuNjLPfZouYw-pdf?v=8632e9d8. Accessed 24 July 2023.
- Application note: Pegasus Prime virus filtration: Robust retention after pressure interruptions (Stop and Start). Bioprocess International 27 Oct. 2016. https://bioprocessintl.com/sponsored-content/pegasus-prime-robust-retention-pressure-interruptions-stop-start/. Accessed 24 July 2023.
CY37201-290324-AN