Introduction to gene therapy viral vector purification
Adeno-associated virus (AAV) is the main vector for gene therapy. Scalable, cost-efficient, and robust filtration and chromatography-based processes are required for AAV purification. Key for a successful process are high overall yields and efficient removal of empty capsids and other impurities.
Overview of AAV purification process
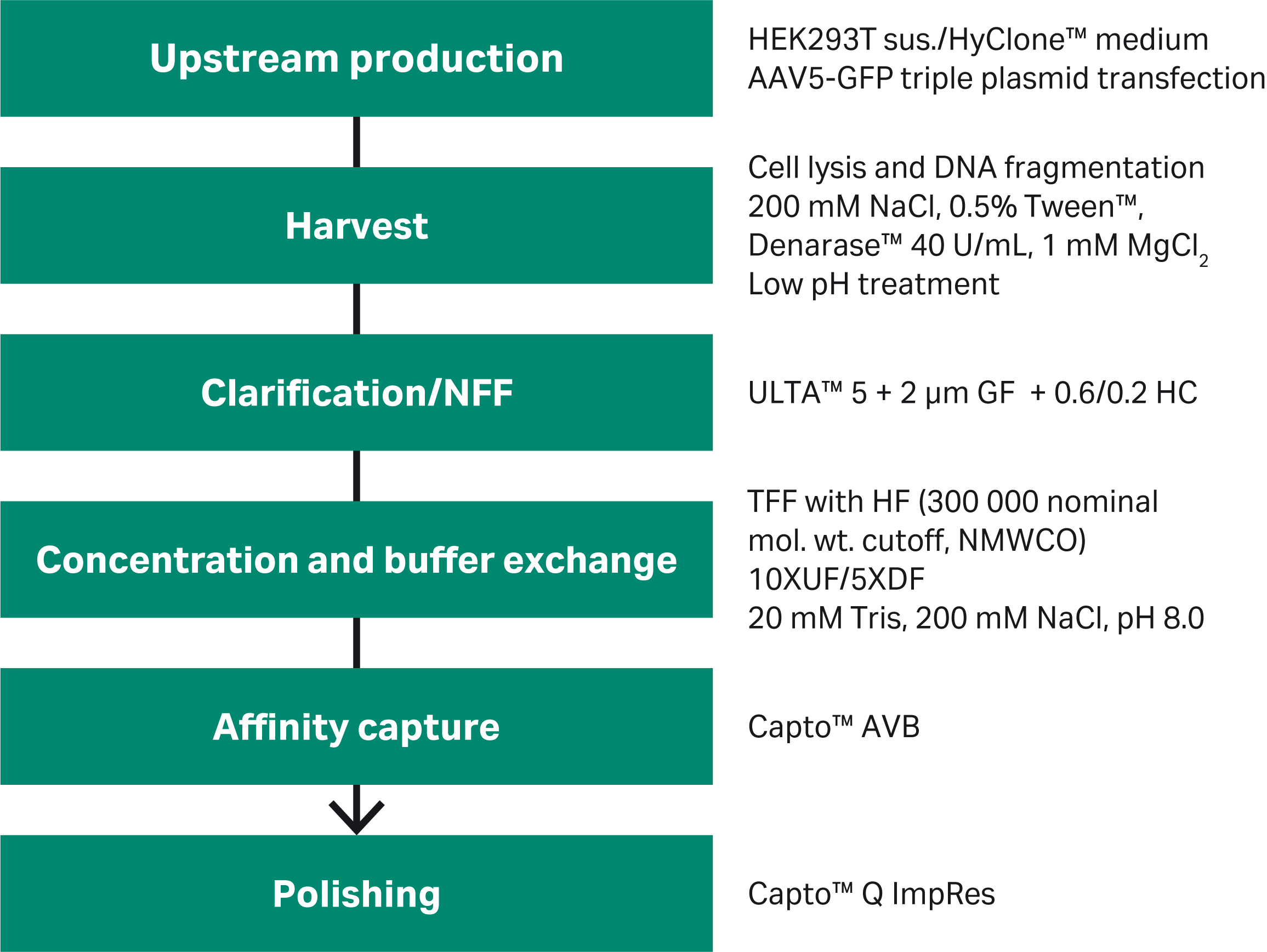
Here, we present results from purification process development of AAV2 and AAV5 serotypes, including harvest by cell lysis, clarification, concentration and buffer exchange, affinity capture and finally anion exchange polishing to reduce empty capsids. We present a Biacore™ surface plasmon resonance (SPR) assay for robust and accurate determination of AAV2 titer. Figure 1 shows an illustration of the developed process.
Fig 1. Overview of the AAV vector purification process for AAV5.
Materials and methods for AAV vector purification and viral titer assay
AAV production: HEK293T cells adapted to suspension culture (CRL-3216 ATCC) were grown in HyClone™ HyCell™ TransFx-H medium (Cytiva) using Xcellerex™ XDR-10 (Cytiva) or ReadyToProcess WAVE™ 25 bioreactors (Cytiva). An optimized triple-plasmid (Rep/cap: helper: transgene GFP) transfection protocol was used to produce AAV2-GFP or AAV5-GFP.
Harvest and clarification: At time of harvest, we lysed cells with 0.5% Tween™ 20 + 150 mM NaCl and treated with 40 U/mL Denarase™ (c-LEcta) + 1 mM MgCl2 for 4 h in the bioreactor. To avoid possible spontaneous precipitations, we treated the feed with pH 4.0 for 30 min before neutralizing to pH 7.0. The resulting precipitate was allowed to sediment and was discarded. The cell lysate was clarified by normal flow filtration (NFF) using a combination of 2 μm and 5 μm GF as well as 0.6/0.2 μm HC ULTA™ filters.
Results
Tangential flow filtration (TFF)
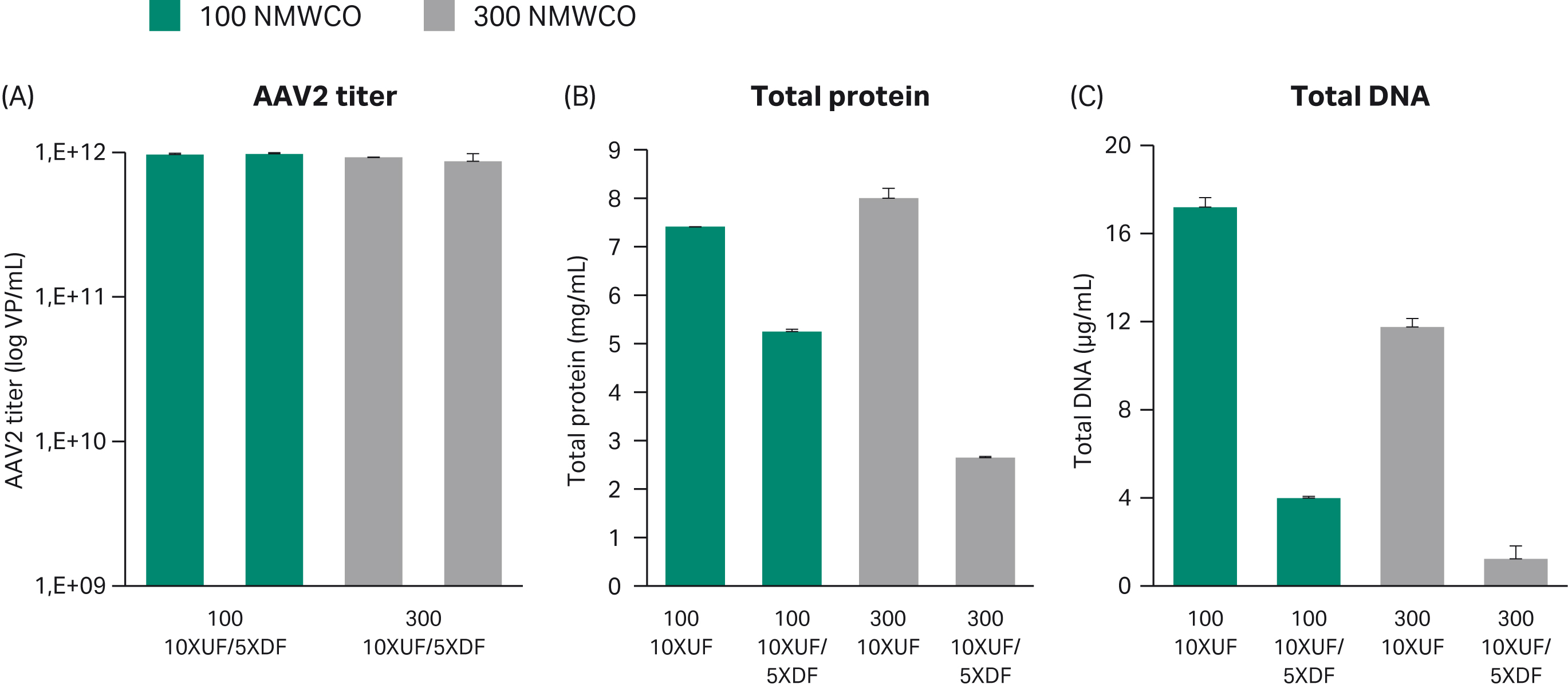
For concentration and buffer exchange of the clarified harvest feed, we compared hollow fibers of two nominal molecular weight cut-offs (NMWCO): 100 000 and 300 000. Recovery of virus was similar, but the 300 000 membrane provided more efficient reduction in total protein and DNA compared to the 100 000 membrane (Fig 2).
Fig 2. Concentration and buffer exchange with TFF of clarified AAV2 feed using ÄKTA flux™ 6 system. (A) The AAV2 titer was determined by ELISA in duplicate. (B) Total protein was analyzed with BCA and (C) total DNA was analyzed with PicoGreen™. In each set of bars, the first bar is after concentration and the second is after buffer exchange into 20 mM Tris, pH 7.8 + 200 mM NaCl. UF = ultrafiltration, DF = diafiltration.
Affinity capture
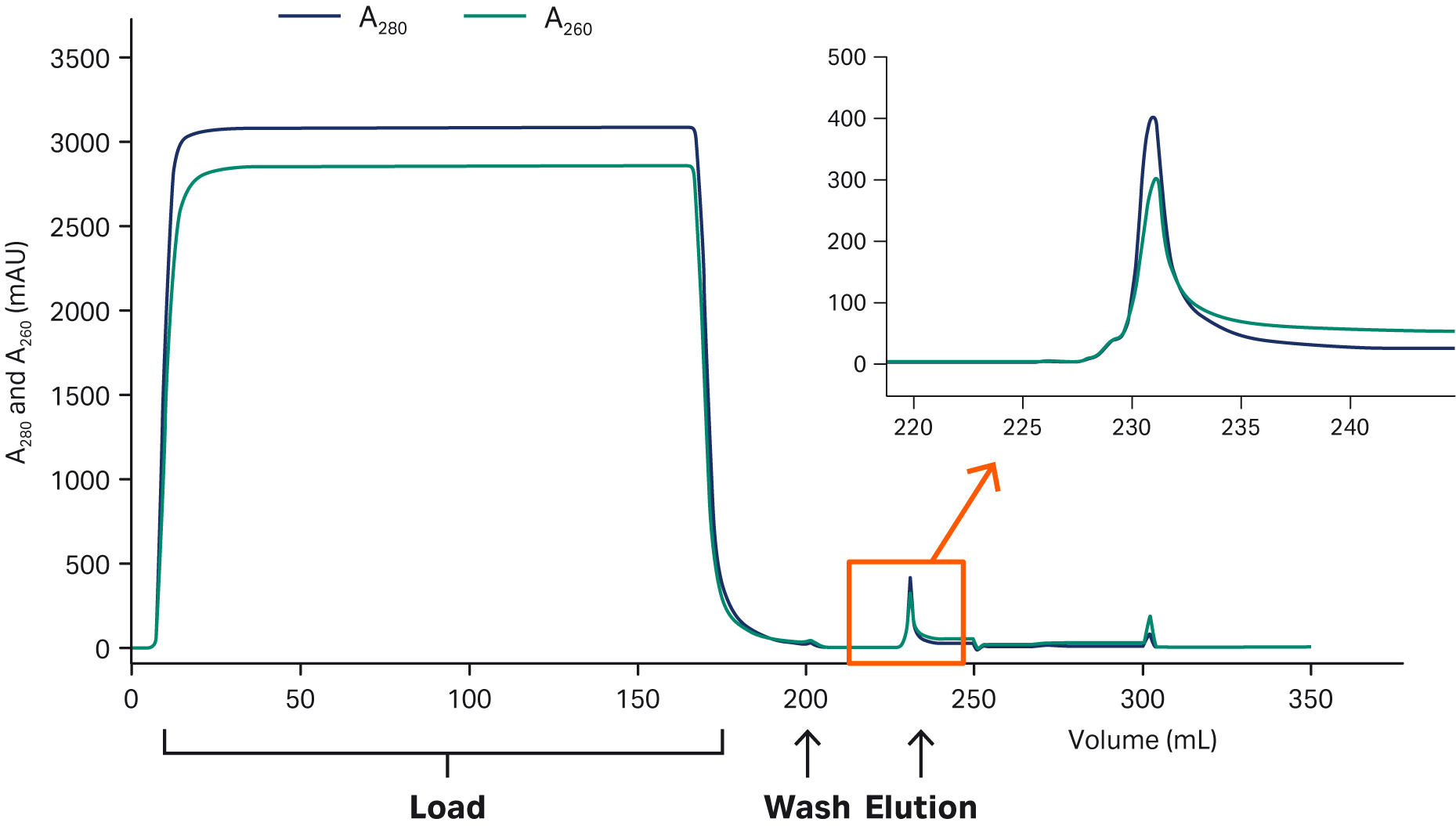
We used Capto™ AVB for affinity capture of AAV2 and AAV5. See Figures 3 and 4 for results. Concentrated feed of both serotypes showed similar Capto™ AVB performance with a binding capacity of approximately 1014 virus particles (VP)/mL and a recovery of ~ 80%. The concentration factor was 100- to 200-fold, depending on the harvest titer. However, AAV5 required lower pH for elution compared to AAV2.
Fig 3. Capto™ AVB affinity capture of AAV2. Capto™ AVB 5 mL HiTrap™ column was run on an ÄKTA pure™ 25 chromatography system. We equilibrated and washed the column in 20 mM Tris pH 7.8 + 200 mM NaCl. AAV2 was eluted in 50 mM citrate pH 3.5, 500 mM NaCl, and arginine. Similar results were obtained with AAV5, which was eluted in 50 mM glycine, pH 2.6. The eluted virus peak is indicated in the chromatogram and shown enlarged.
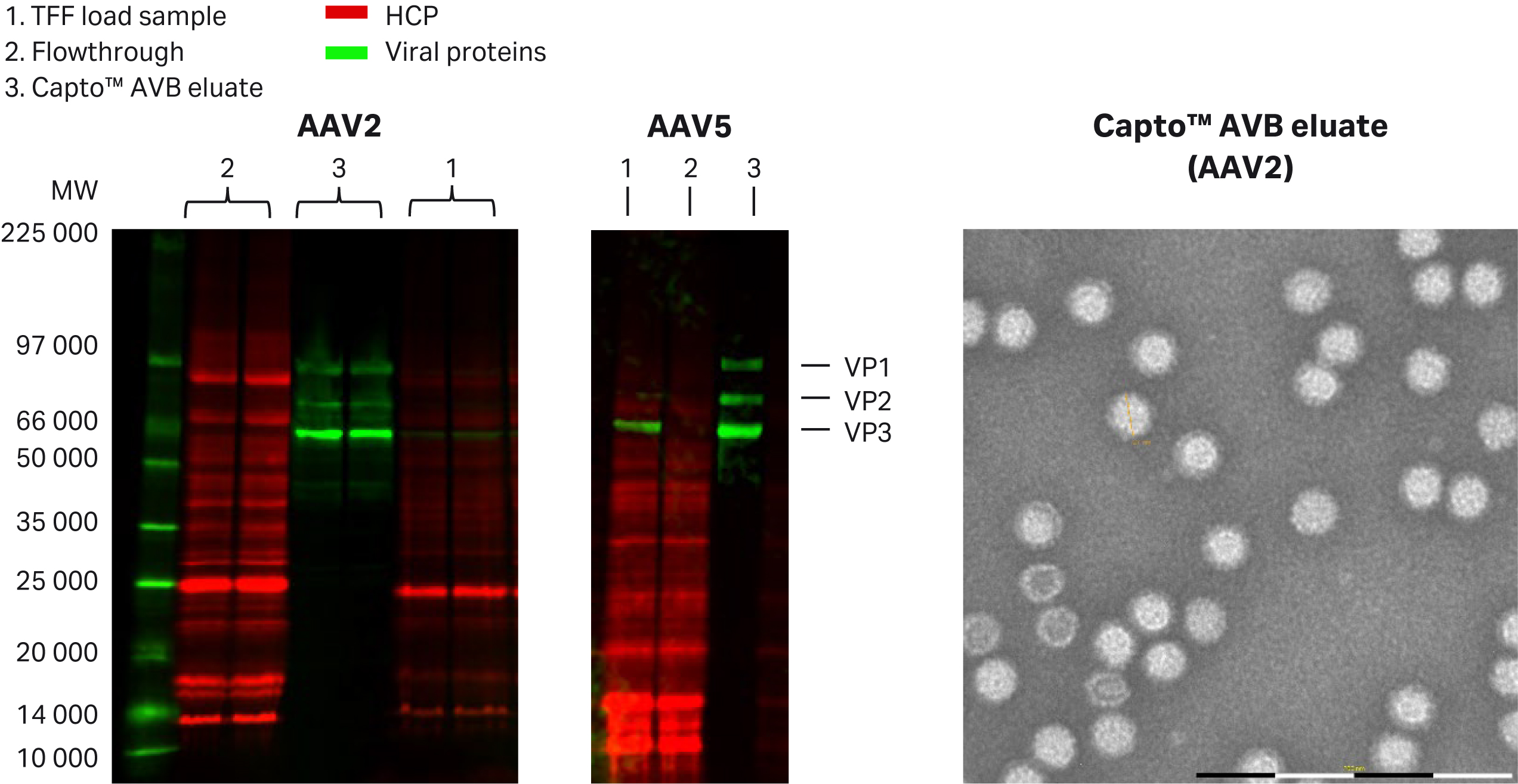
Fig 4. Analysis of Capto™ AVB fractions. Multiplex fluorescence (Cy™5 prelabeled and Cy™3- labeled secondary antibody) SDS-PAGE, and Western blot analysis showed absence of detected virus in the flowthrough fractions as well as absence of HCP in the eluate. TEM analysis of eluate (MiniTEM™ system, Vironova AB, negative stain) showed that the AAV capsids had expected size and high purity. The empty particles are likely to be stained (dark coloring inside capsids).
Polishing to enrich full capsids
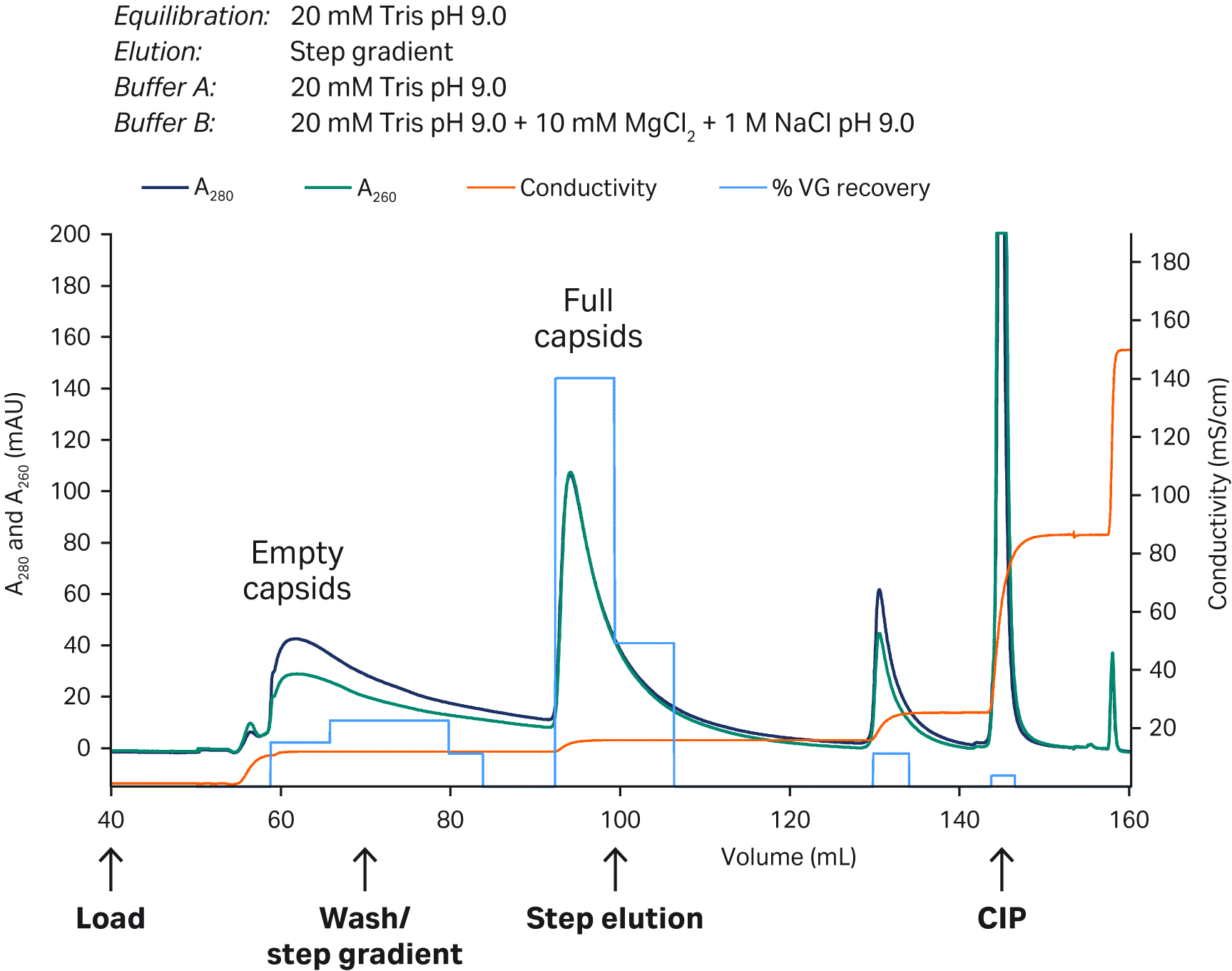
Full capsids containing viral genomes have a lower pI compared to empty capsids (approx. 5.9 vs 6.3) and have a higher total charge. This difference can be used to separate full and empty capsids, where full capsids elute at higher salt concentrations with anion exchange resins. We used a Capto™ Q ImpRes HiScreen™ column for polishing at pH 9.0. We eluted with a combination of NaCl and MgCl2, which allowed full capsids to be enriched (> 50%) with good recovery. Peak fractions (Fig 5) were analyzed with qPCR (viral genomes, VG) and ELISA (viral particles, VP) and confirmed the increased A260:280 ratio indicating full capsid enrichment. We are currently fine-tuning the conditions and gradients as the next step to maximize the performance.
Fig 5. Capto™ Q ImpRes column was equilibrated in 20 mM Tris pH 9.0 (buffer A) and a diluted Capto™ AVB eluate was applied. Elution was performed with a step gradient of 11%, 15%, and 25% B buffer (20 mM Tris pH 9.0, 1 M NaCl, 10 mM MgCl2). The peak with enriched full capsid had a significantly higher A260:A280 ratio and eluted at higher salt concentration as expected. The bulk of viral genomes (VG) were recovered in the full capsid peak.
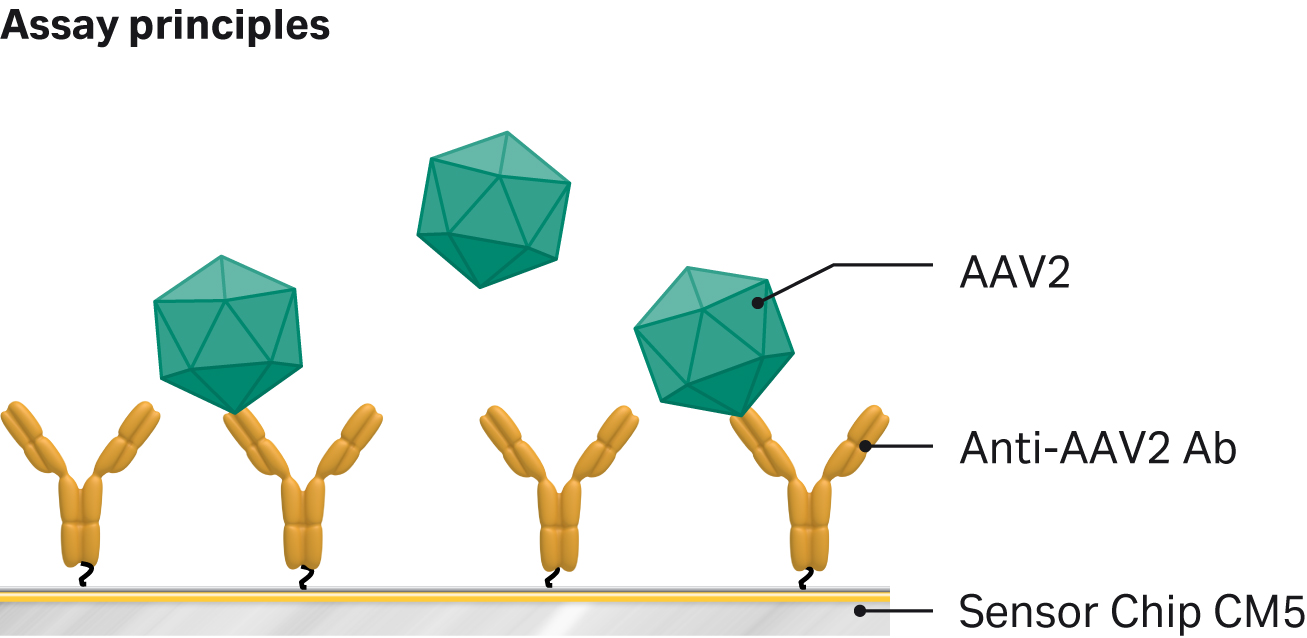
Robust and reproducible AAV2 viral titer assay using Biacore™ SPR systems
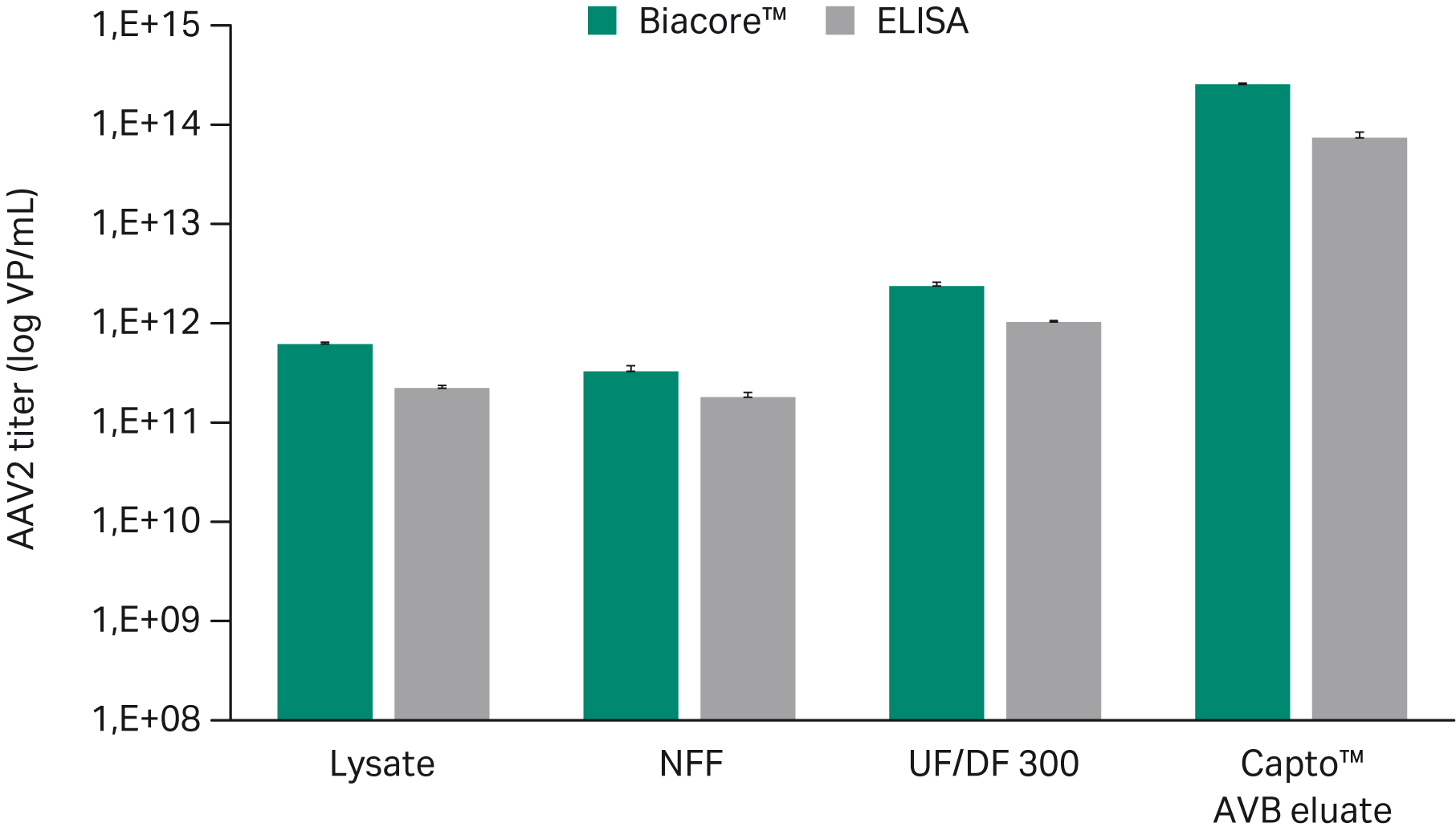
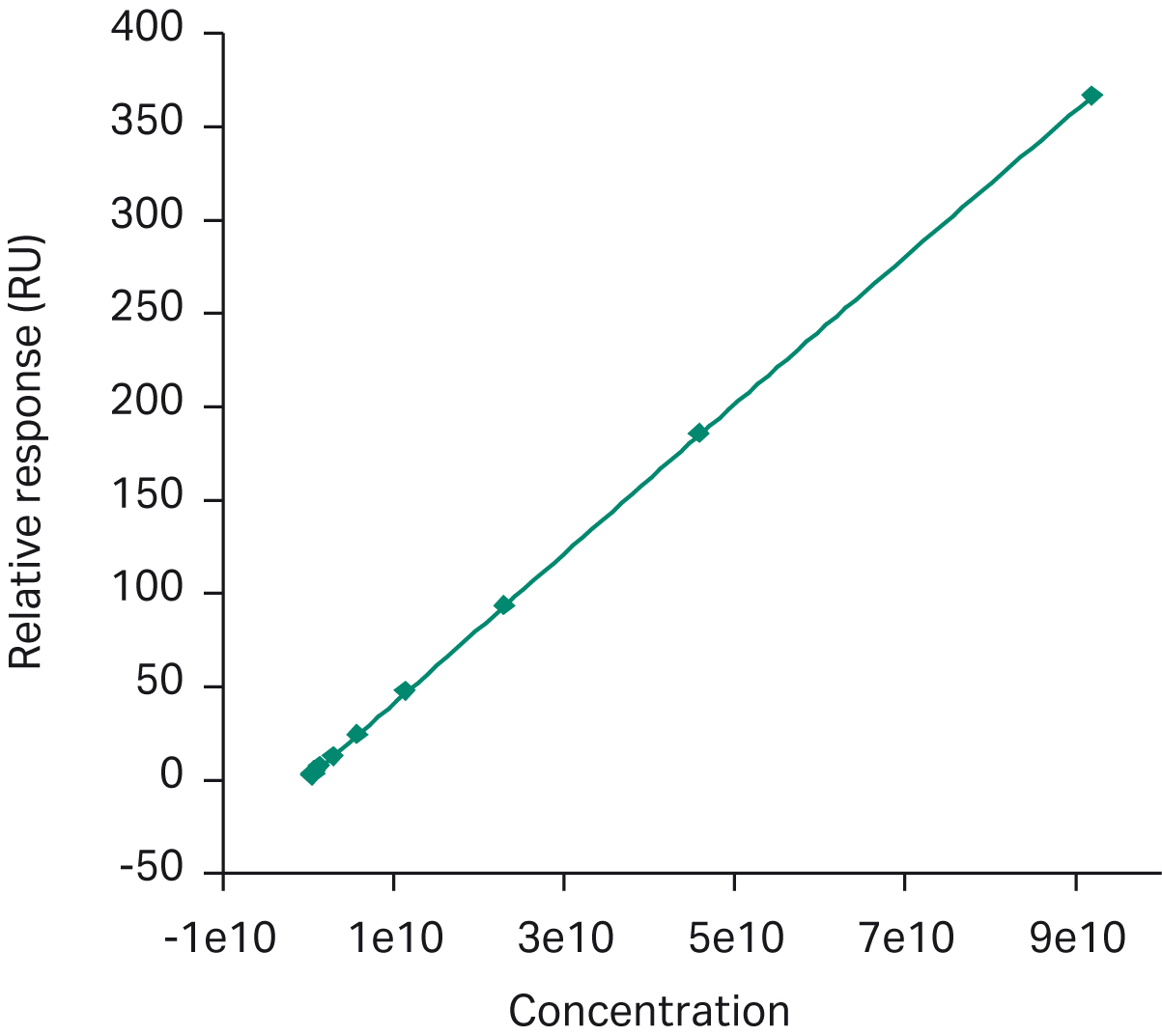
A Biacore™ SPR assay was developed with an anti-AAV2 antibody immobilized to the sensor chip. We injected samples over the surface, and a binding response signal was used to calculate the capsid titer using a calibration curve. Calibration range was approximately 108 to 1010 with an limit of detection (LOD) of 2 × 109 VP/mL. Coefficient of variation (CV) was typically < 5%. The assay was accurate and robust with a higher degree of automation compared to an ELISA assay (Fig 6 and 7)
Fig 6. Biacore™ AAV2 assays were compatible with samples from different process steps. Results were comparable to the traditional ELISA assay.
Fig 7. Biacore™ AAV2 assay for total capsid titer. The calibration curve was produced with the ATCC AAV2 reference standard.
Conclusions
Here, we show optimization of AAV2 and AAV5 purification process steps and a convenient SPR-based viral titer assay with high performance. Based on the results presented here we conclude:
- TFF with 300 000 NMWCO hollow fiber provided good AAV recovery and better impurity removal compared to 100 000 NMWCO
- Affinity capture chromatography with Capto™ AVB was efficient
- Anion exchange polishing with Capto™ Q ImpRes enriched full capsids > 50%. This step may require optimization, depending on the AAV vector serotype
- A new Biacore™ assay was accurate and robust for AAV2 quantitation and was more automated than an ELISA assay
Learn more about solutions to make AAV viral vectors for gene therapy.