The development of cell therapies is changing healthcare. There are thousands of patients around the world who have new hope for a positive disease outcome thanks to the increasing availability of these treatments. The vein-to-vein workflow for these therapies, however, is not without challenges, many of which will increase as we scale up to treat more patients. Here, we consider the challenges and risks associated with the cryogenic supply chain for cell therapies and provide considerations for mitigation strategies.
The typical autologous cell therapy workflow includes a cryogenic logistics cycle that involves the clinic or hospital where a patient will receive treatment and the manufacturer producing the therapy. A biopsy or sample is collected from the patient, engineered into a therapeutic product, and then reintroduced to the patient for treatment. By comparison, allogeneic cell therapy involves fewer transit legs, as it uses a single donor sample for engineering cell therapies for multiple patients. In either case, maintaining cell viability throughout these transit steps is essential, and any delays or disruption could put that viability at risk, along with treatment efficacy and patient safety.
If cells lose viability after collection, the manufacturer might have a difficult time developing the therapeutic product and could potentially need a new sample, which is not always available. Treatment efficacy is at risk if the viability of the therapeutic product is compromised in transit from the manufacturer to patient, potentially leading to the need to start over. In either case, the patient’s health is at stake and there are substantial financial implications for the manufacturer, given these therapies can cost more than $100 000 per patient to produce.
While current approaches may be capable of managing the existing volume of cell therapy shipments (though not without challenges), scaling these treatments up and out will increase pressure on existing cryogenic logistics networks. Considering and addressing these challenges early in a therapy’s development could help reduce the risk of future supply chain disruptions and make sure patients continue to receive the right treatment, of the necessary quality, at the right time.
Why cryopreservation below -120°C is beneficial, and necessary
As the benefits of living cell treatments become apparent, so do the challenges of their transport and storage. Unlike freeze-dried or chemically synthesized treatments, biological samples for cell therapies are alive and active, undergoing disruption and metabolic decline once removed from the body.
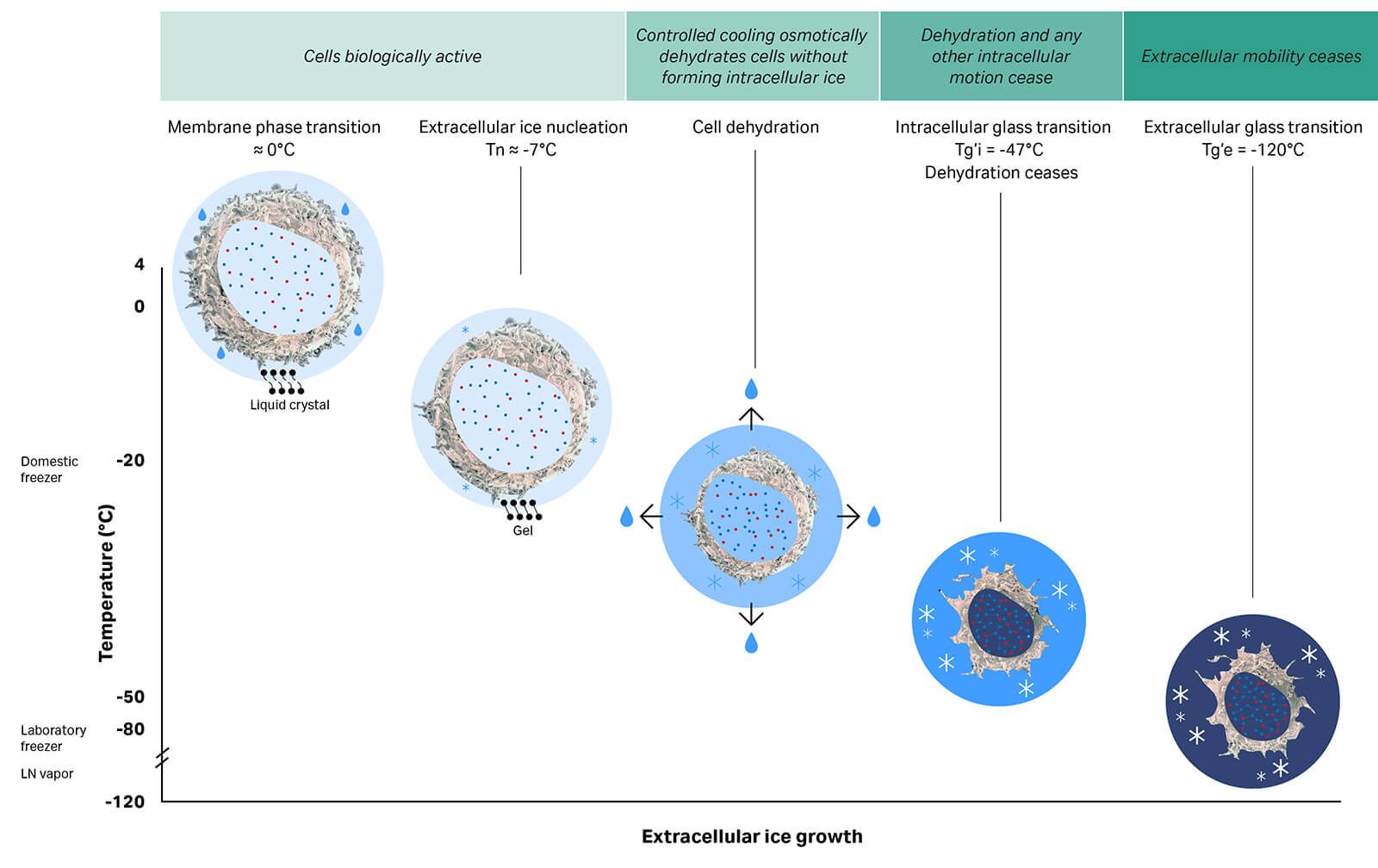
Short-term storage at 4°C is possible in some instances, such as transporting a sample to a manufacturing facility adjacent to the collection site. Longer distances, however, require cryopreservation and the continued maintenance of temperatures below -120°C — the point at which molecular mobility within the sample becomes restricted to vibrations of atoms or bonds and reorientation of small groups of atoms1 — and the risk of degradation over time is greatly reduced, as depicted in Figure 1. Preserving samples by keeping them below this temperature is key to successful storage for extended periods.2,3
Fig 1. Cryopreserving cells. Controlled cooling dehydrates cells with minimal damage from ice crystal formation. Dehydration proceeds until the intracellular space vitrifies (intracellular glass transition). With further cooling below -120°C, the extracellular glass transition stops residual molecular mobility and enables stable long-term cryogenic storage and transport of cells. (Figure adapted from Meneghel et al. 20192)
To maximize cell viability, increasing the sample temperature above -120°C should only be done in a controlled manner by the cell therapy manufacturer or at the point of care. Outside of a controlled thawing environment, such as during storage or transport, increasing a sample’s temperature higher than -120°C will damage the cells, affecting the quality and quantity of those that are viable. Uncontrolled warming could introduce inconsistency between cryobags or batches, and even destroy samples altogether.3-5
The cryogenic cold chain within an autologous cell therapy workflow
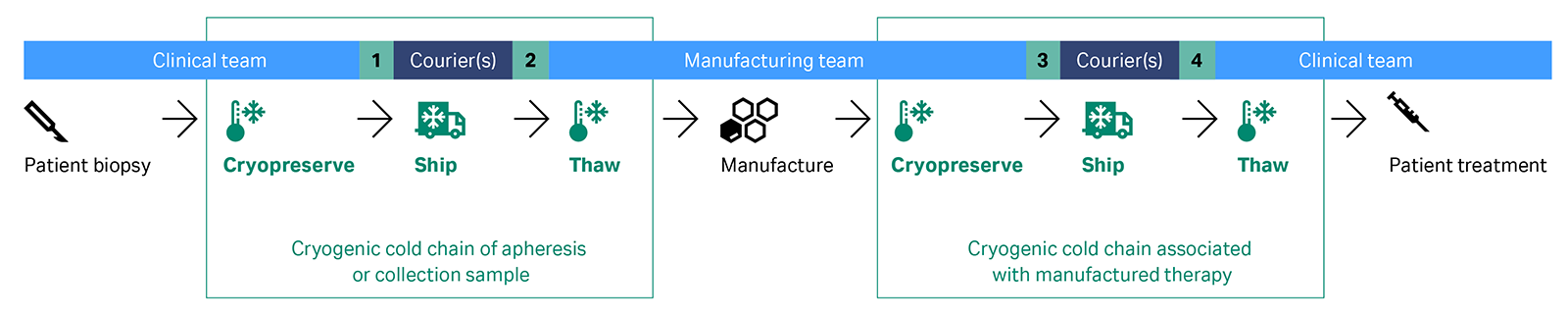
When dealing with autologous chimeric antigen receptor (CAR) T-cell and tumor infiltrating lymphocytes (TIL) therapies, the final products and, in some cases, starting materials require cryopreservation. This process involves several critical steps: the freezing, cryogenic shipping, and thawing of a cellular material, all essential to the maintenance of cell viability. In summary, clinical staff take a biopsy or apheresis product from the patient. If cryopreservation is deemed necessary, the clinical staff may oversee the process and ship the sample to the manufacturer in a liquid nitrogen-based dry shipper. Alternatively, the manufacturer may handle cryopreservation. After cryopreservation, the manufacturer thaws the cellular material when a manufacturing slot becomes available. Next, the manufacturer isolates T cells and engineers, expands, and formulates them into a cell therapy. Lastly, the treatment is cryopreserved and sent back to the clinical site for thawing and administration, as illustrated in Figure 2.
These two supply chains — donation for therapy manufacture and transport of the therapy back to the patient for infusion — are complex, with the cryopreserved cell samples and treatments changing hands multiple times. Throughout the shipment process, careful handling and record management are needed to maintain sample quality and document transit information. With treatment applications for cell therapies rapidly expanding, not all stages of this workflow are primed to scale in their current form.
Fig 2. The vein-to-vein autologous cell therapy workflow involves at least 3 parties (clinical, manufacturing and courier) to deliver the cryogenic cold chain processes, with at least 4 points of transfer during the shipping (at points 1-4) and potentially multiple more if shipment requires a network of carriers (denoted term ‘Courier/s’).
The key risks and challenges relating to cryogenic logistics
The primary goal of cell therapy logistics is to safeguard samples through every step before, during, and after transportation, so effective treatments can be delivered to patients in need. Cryogenic shipping vessels are designed to maintain samples at cryogenic temperatures and avoid sample damage. Couriers play an important role in the safeguarding process, while also ensuring handling requirements are adhered to en route and that contingency plans are in place to manage any unexpected incidents or delays. Together, the aim of a partnership between a cell therapy manufacturer and a courier is to efficiently manage logistics while remaining compliant with international Good Distribution Practices (GDP) and preparing to scale effectively.
Cryogenic cold chain packaging requirements
Each cell therapy shipment involves several layers of packaging, designed to provide product integrity and stability:
- Primary packaging: cryobags or cryovials that contain the cell sample
- Secondary packaging: container or rack for securing the primary packaging
- Tertiary packaging: liquid nitrogen (LN2) dry shipper, also sometimes called a dry vapor shipper
- Outer packaging: any pallet or box to contain and secure the LN2 shipper
Until recently, the tertiary packaging for shipping at cryogenic temperatures included only pre-conditioned LN2 dry shippers, shipped empty to the hospital or manufacturer and then with payload held in the vapor phase to the intended destination. While the LN2 is at -196°C, the vapor phase gradually vents. Most dry shippers can maintain a temperature below -150°C for between 5 to 10 days under normal conditions, which is enough to keep the cellular material below the critical -120°C threshold. This period is known as the hold time. A data logger or smart monitor captures the temperature, tilt, and location of the container throughout transit. This journey can span days, especially in cases requiring air freight across international borders.
Suitable packaging and a carefully planned route are essential for each shipment. The physics of LN2 vapor, however, presents many challenges and creates constraints throughout the logistics process. Managing dry shippers requires complex preparation, charging, and validation processes:
- First, each shipper needs thorough cleaning of the fibrous zeolite matrix used to hold the LN2, as this can degrade with use, reducing the shipper’s hold time.
- The shipper is then pre-charged with LN2 so it can provide the predicted hold time required for its journey.
- Charged dry shippers are weighed to assess their readiness and filled to meet the fully charged weight.
- Between every shipment, a visual check is completed to validate the shipper’s condition, as damage to the seal around the neck of the shipper or denting of the exterior can reduce the hold time.
- To check that a dry shipper is performing and certify the expected hold time, a regular “24-hour evaporation validation” test is completed between shipments.
These steps do not guarantee the shipper’s hold time, as an incident like tilting during transit increases the rate at which the LN2 vents, introducing risk of the -120°C threshold being exceeded before the shipment reaches its destination. Specialty couriers might transfer the sample to a backup pre-charged dry shipper, removing the risk of uncontrolled thawing but introducing a break in the sample’s chain of custody and identity.
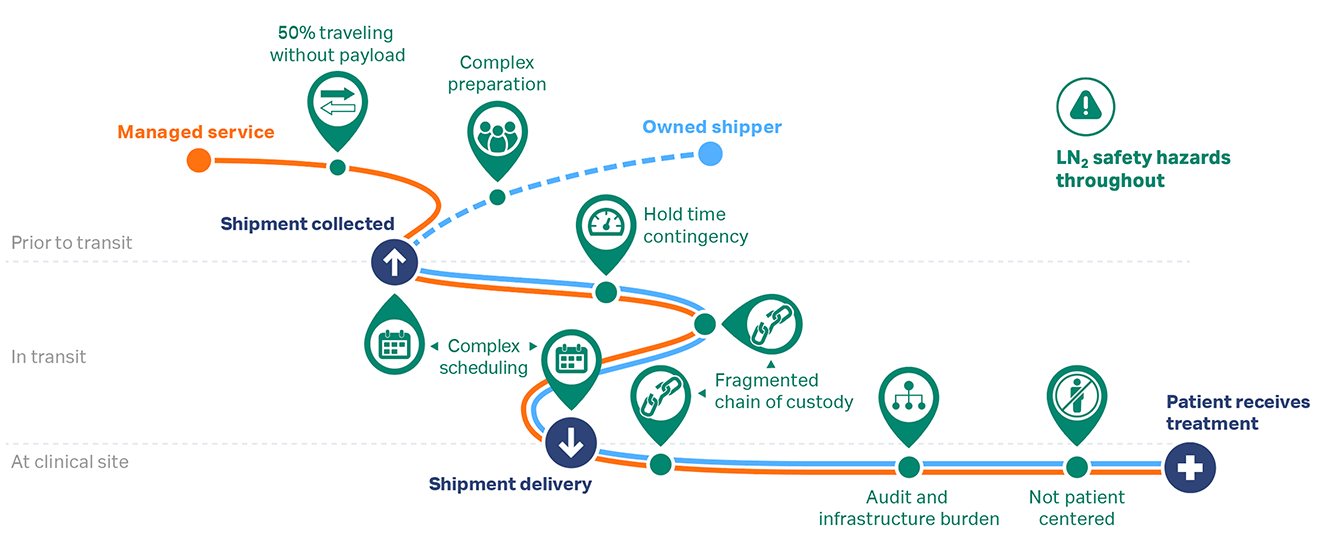
As a result of these inherent risks, there is a need for LN2 infrastructure throughout the cryogenic cold chain — at the courier’s charge-hub, the manufacturing site, en route, and at the clinical site — with associated hazard management training and plans. All parties are also under scheduling constraints to coordinate the timing of the clinical and manufacturing sites’ workflow steps with the dry shipper’s preparations and rigid logistics timetable, as illustrated in Figure 3.
Fig 3. Considerations for cryogenic logistics (green) when shipping with courier supplied pre-charged shipper (orange) or using shipper from cell therapy manufacturer’s fleet (blue).
The potential challenges related to dry shippers have led to the development of LN2-free systems using an electrically-powered cryocooler. These electrically powered shippers are cooled to cryogenic temperatures below -190°C using a Stirling engine-based cryocooler unit and then sealed for transport, providing a comparable hold time to LN2 shippers without many of the challenges associated with using LN2 for cooling.
Table 1 lists some of the key risks in the cryogenic cold chain, as well as causes and possible consequences.
Table 1. Key risks, causes, and consequences associated with cryogenic shipping
| Risk |
Possible causes |
Consequences |
| Precise hold time of LN2 dry shippers cannot be guaranteed | Variable LN2 evaporation rate between dry shippers, even those of the same model | Uncertainty or an overestimation of hold time can lead to a temperature excursion over the critical -120°C threshold, damaging the cell samples. |
| Difficulty in accurately determining amount of LN2 stored | ||
| Zeolite sponge degradation, reducing ability to hold LN2 | ||
| Vacuum seal in dry shipper’s neck degrades, reducing insulation efficiency | ||
| Unexpected and undetectable tilting, increasing rate of LN2 vapor loss | ||
| Physical damage to, and contamination of, samples | Unexpected shocks during transit causing damage to the cryogenic shipper packaging and the fragile cryobags (primary packaging) within | Any physical damage to the primary packaging (cryobags) during loading, unloading, and transit might render the cell therapy less effective or lead to loss, if ruptured. Handling and damage introduces the risk of contamination from microbes held frozen within the stored LN2. |
| Multiple points of transfer of cryobags into and out of the container to a backup shipper or during loading and unloading, increasing opportunities for damage | ||
| Fragmented chains of custody and identity | Multiple shipper handovers within the transport network | Loss of chain of custody, or the associated records, might mean clinical or scientific staff are unable to verify quality requirements were met. Loss of chain of identity could result in a mix up of treatment or delivery to the wrong patient. |
| Sample transfer to backup dry shippers or to temporary cryogenic storage on-site | ||
| Fragmented nature of multi-party, multi-system records leading to delays in receiving shipment or loss of shipment documentation | ||
| Lack of training of personnel within the transport network | ||
| Differences in SOPs or variation in rigor of how SOPs are applied across geographies | ||
| Transportation holdups | Unexpected delays, such as weather events | Loss of or damage to cellular sample if contingency plan is not in place to maintain the sample at cryogenic temperatures. Contingency plans could include rerouting the shipment, using a pre-charged backup LN2 shipper, or, if using an LN2-free option, putting the shipper on electronic charge. |
| Customs or paperwork issues: incorrect/ incomplete paperwork due to human error | ||
| Unplanned rescheduling of therapy administration to patient leading to a need for temporary storage at or near the clinical site | ||
| Scheduling complexity | Efforts to maximize LN2 hold time dedicated to transport legs | Complex scheduling logistics put pressure on manufacturing workflows and the patient’s treatment schedule must coincide with the dry shipper’s arrivals and dispatch. Limited access to clinical site could require local temporary storage. |
| Transportation holdups | ||
| Clinical site might have limited or no storage for shippers | ||
| No access to temporary cryogenic storage facilities at clinical site |
SOP = standard operating procedure
Mitigation strategies for cryogenic supply chain risks
Quality planning and processes optimize outcomes
Cell therapies are manufactured to GMP standards and should be transported according to GDP requirements (Fig 4). Maintaining compliance with GDP extends the quality assurance delivered by GMP through the entire logistics workflow. Ensuring logistics adhere to GDP guidance does not guarantee that there cannot be failures, but rather helps identify, manage, and de-risk cryogenic transport.
A cell therapy manufacturer might specifically look for GDP certification for peace of mind and as a sign of quality assurance. Generally, GDP aims to ensure:
|
Without GDP compliance, there is risk of cryogenic shippers and cryopreserved cellular samples being unintentionally mishandled and paperwork not being correctly maintained. What might seem like small oversights at the time could have a significant impact on cell therapies and their recipients, especially if the chains of custody and identity are broken. Many of the potential issues would not be evident until the container reaches or fails to reach its destination.
Risk management strategies
Each shipment is unique, but there are common points to focus on when working to eliminate or mitigate risk to sensitive and precious cargo. With more packing and courier service options for cryogenic shipping becoming available, there is now a range of mitigation strategies to consider. Table 2 lists cryogenic logistics strategies to manage the critical temperature of cell therapies prior to shipment, during transit, and at the delivery site.
Table 2. Temperature excursion risk mitigation strategies
| Requirement / strategy | ||
| Stage | Packaging considerations | Courier selection |
| Prior to shipment | When using a LN2 shipper, build in extra hold time to allow safety margins for empty transit to sample collection point, wait time at the manufacturer, or any other unexpected delays during transit (due to the weather, long customs clearance, etc). Use an LN2-free electrically cooled shipment system that can be held on charge at the manufacturing location or clinical site prior to or following sample loading so hold time is only required for shipment leg(s). |
Use a courier with experience and expertise in cryogenic logistics that is knowledgeable about all packaging options and able to optimize routes based on cargo, not carriers. If choosing a courier-supplied pre-charged dry shipper, request certification to confirm anticipated LN2 hold time can be met (complex cleaning, validation tests, and charging process). |
| During transit |
Use a cloud-connected monitoring and alert system to continuously track shipper conditions (including temperature, GPS position, and tilt). Choose a smart LN2-free shipper to provide continuous visibility of remaining standby time and performance throughout the shipment. Minimize tilt by bolting the dry shipper to a large shipping pallet or by using a LN2-free shipper with low center of gravity and regular dimensions. |
You must be able to ensure all personnel in the logistics network are trained to handle cryogenic shippers and deliver GDP standards. Implements SOPs across the transit network, (e.g., handling requirements are systematically applied by all carriers across the globe). A courier should be prepared to minimize unpredictable transit delays (e.g., can flex lanes and freight carrier or is proficient at expediting processing through customs hold-ups). |
| Work with a partner who has established technology and expertise in maintaining samples at cryogenic temperatures within hubs (e.g., transfer of sample to pre-charged LN2 back-up shipper or electrical charge of LN2-free shippers). | ||
| At the clinical site or on delivery |
Use smaller packaging that can be maneuvered for ease of access to and within the clinical site. Make sure there is appropriate infrastructure for temporary cryogenic storage of samples, either in maintained on-site freezers, within the dry shipper, or through an electrical charge point for an LN2-free system. |
|
| A courier should have an optimized schedule for “just in time” delivery. | ||
Currently, it is common to allow at least a 30% hold-time margin for an LN2 dry shipper to account for temporary storage during wait periods prior to or following delivery. In the short-term, continuing to use a larger shipper to extend hold time might be the easiest option; however, some clinical sites cannot store or internally maneuver large dry shippers (particularly if secured to a pallet), potentially and unintentionally limiting the clinical reach of a therapy.
The required training and development of additional hazard management protocols can also deter or delay the enrollment of clinical sites not accustomed to working with LN2. It might, therefore, be easier for some sites to pursue LN2-free options. It is important to note that all risk mitigation strategies have trade-offs, so it is vital to take care in defining and prioritizing short- and long-term logistics requirements.
Mitigating other physical, data integrity, and regulatory risks
As the logistics risks extend beyond the challenge of maintaining the sample below -120°C, there are other physical and regulatory elements that also need consideration when outlining requirements, mitigation approaches, and trade-offs.
Gone are the days when bubble wrapping a sample prior to loading into a dry shipper was deemed suitable for secondary packaging. Now, there are a variety of packaging options to protect a frozen sample from rupture. Any strategy aimed at minimizing physical damage must also limit sample transfers between transport and storage containers. Limiting transfers minimizes the risk of breaks in the sample’s chain of custody and identity. Some approaches include:
- Using the shipper for temporary cryogenic storage during delays or at the clinical site: This strategy is achievable with LN2 dry shippers if there is adequate hold-time provision or for a period of several weeks with an LN2-free shipment system connected to a standard electrical power outlet.
- Employing a process management mitigation strategy: Define clear SOPs for all involved parties to implement when loading and unloading samples to/from shippers (including couriers supplying backup LN2 dry shippers) or into storage facilities.
Digitalization for data integrity
Until recently, shipment records have focused on logging the shipper’s conditions throughout transit. Now, newer cloud-based platforms can automatically secure data digitally, removing reliance on clinical teams to upload data logger readouts or manage paper records. This approach removes the risk of potential validation record loss, while also providing real-time information on the shipment.
Integrating condition data into the batch record requires an additional data transfer step from the courier to the manufacturer’s systems. Selecting a cloud-based system that automatically uploads the condition information to the cell therapy manufacturer’s own record system helps ensure uninterrupted flow of information through a secure process with strong data integrity.
On arrival at a clinical site, any transfer of sample to temporary on-site storage raises the issue of needing to validate the storage processes and equipment. Stakeholders must also be able to access the cryogenic temperature record of the sample while it’s held in the freezer. Data collection here remains a challenge for clinical site staff — a challenge that could be easily overcome using a smart shipper (LN2 or LN2-free) for temporary storage, producing a continuous digital condition record until just prior to treatment. Paper records can be eliminated by providing manufacturing and clinical teams with a digital SOP tool to direct and capture a fully recorded loading or unloading process. Including a step to read the sample’s barcode, the shipper’s identification barcode, and a barcode on the specific shipment’s transportation documents enables personnel to verify the chain of identity and link this record to the shipper’s service and condition records, verifying that the right sample reached the right destination.
Chain of transfer records are a standard part of most courier services, documenting every stakeholder responsible for handling the shipment. Linking this record to the shipment’s condition information can be useful for identifying any weak links where a package handling SOP needs refining or where a person might require further training. Having a sample’s complete electronic shipment record — including logistics workflow SOP records, shipper service history, transit condition data, and chain of custody records — optimized, digitally recorded, and fully integrated offers a valuable tool to drive improvements in logistic processes and forms management.
Determining a strategy for managing the complex risks associated with cell therapy supply chains requires calculation and consideration. A single approach might not be suitable in all instances. Flexible, multi-strategy logistics, however, could account for differences between the infrastructure and scale at clinical sites, supporting an ideal outcome for manufacturers, providers, and patients.
The impact of future scaling of cell therapies
All the risks discussed here can increase with scale. As the availability of cell therapies grow, so will global demands on the manufacturing workflow. How can we adapt the cryogenic supply chain to address these increasing pressures?
Developing partnerships
A strong partnership between the cell therapy manufacturer, courier, and clinical teams is critical to reliably delivering cell samples. This partnership can be reinforced by technology, packaging, and digital systems to deliver processes at each step and across global logistics networks that are consistent and controlled.
Standardization of workflow and infrastructure
Using LN2-free controlled-rate freezers and LN2-free shipment systems eliminates LN2 from cryopreservation, shipping, and temporary storage. This transition would also remove associated recurring costs, such as LN2 hazard training for clinical teams. Finding opportunities to automate and standardize elements within the supply chain will further help to produce cell therapies at a cost that healthcare markets can afford, driving more widespread adoption. Taking a broader perspective and looking at cellular shipments within the context of the vein-to-vein workflow can help identify additional efficiency opportunities. For example, a complete electronic shipment record system that automatically integrates with the digital batch record can improve productivity in the data management processes.
Viewing the cryogenic cold chain as a cohesive collection of workflow steps rather than considering logistics as an isolated activity will unlock other cost containment opportunities. For example, the cryocontainer used for cryopreservation of the patient’s initial sample often varies between clinical sites. Standardizing this primary packaging could streamline hospital-to-manufacture shipment specifications. Adopting cryovials for cryopreservation of large sample volumes would establish a primary packaging type that is more robust and resistant to rupture than cryobags, redefining secondary packaging specifications and offering potential cost benefits. Assessing this high-level view to identify how processing decisions impact later workflow steps is challenging and often inhibited by an organization’s internal silos and accounting structures, but can provide real gains in efficiency.
Addressing the environmental impact of logistics
An additional consideration of scaling up cell therapies is the associated supply chain’s carbon footprint. At current shipping volumes, the carbon footprint might be more of a secondary concern. However, with scale-up, the multi-leg shipment process means the carbon footprint grows with patient numbers. Again, this is a challenge that could be addressed today by considering how logistics networks can adapt to use LN2-free systems with electrical charging at the clinical site, enabling an efficient two-way shipment cycle from clinics with large patient numbers and ensuring all shipment legs carry a payload.
Summary and conclusions
Cell therapies are a substantial step forward in personalized medicine, offering critically ill patients new hope. The demand for CAR-T cell, CAR-natural killer cell, tumor-infiltrating lymphocyte, and other cell therapies currently in clinical trials will grow, requiring a scale-up of manufacturing and administration. This growth will put increasing pressure on cryogenic logistics within the vein-to-vein workflow.
Those making decisions around cell therapy logistics must take into consideration the multiple risks mapped out above when planning and executing a shipment. From the need for precise scheduling and associated effects on manufacturing and clinical teams to the parameters required to ensure and monitor that cryogenic temperatures are maintained, every step is crucial.
While LN2 dry shippers have been the most common cryogenic shipping vessel option thus far, couriers and logistics processes have adapted to effectively manage the inherent constraints posed by LN2. Here, we have identified some of the key risks involved in the cryogenic supply chain, as well as new risk mitigation strategies that logistics teams can employ. Though all supply chain approaches require multi-party collaboration and GDP compliance, the future could be in LN2-free systems that reduce or eliminate multiple risks associated with current LN2-based container shipping.
As cell therapies become more commonplace, hospitals, manufacturers, and couriers will need to adapt to increasing demands, the potential for global disruptions, and the absolute need to make sure — through their efforts — that future patients receive the right treatment, of the necessary quality, at the right time.
Discover LN2-free shipping that supports logistics risk mitigation strategies.References
- Roudaut G, Simatos D, Champion D, et al. Molecular mobility around the glass transition temperature: a mini review. Innovative Food Science & Emerging Technologies. 2004 Jun 1;5(2):127–34.
- Meneghel J, Kilbride P, Morris JG, Fonseca F. Physical events occurring during the cryopreservation of immortalized human T cells. PLoS One. 2019;14(5):e0217304. Published 2019 May 23. doi:10.1371/journal.pone.0217304.
- Meneghel J, Kilbride P, Shingleton W, Nancekievill A, Morris GJ. Shipping temperatures for cell therapies. ISCT 2020. Poster CY-12874-13MAY20-PT JB80011US.
- Germann A, Oh Y-J, Schmidt T, et al. Temperature fluctuations during deep temperature cryopreservation reduce PBMC recovery, viability, and T-cell function. Cryobiology. 2013 Oct;67(2):193-200. doi: 10.1016/j.cryobiol.2013.06.012.
- Pogozhykh D, Pogozhykh O, Prokopyuk V, et al. Influence of temperature fluctuations during cryopreservation on vital parameters, differentiation potential, and transgene expression of placental multipotent stromal cells. Stem Cell Res Ther. 2017 Mar 11;8(1):66. doi: 10.1186/s13287-017-0512-7.