Cell and gene therapies show promise as effective treatments, but there are some barriers to scaling them beyond the small numbers of patients they reach today. The supply chain and logistics for these advanced therapies are especially critical to ensure the right patient gets the right treatment at the right time. Collaboration is the key to solving challenges such as data management and process variability, as stakeholders work towards a common goal—getting these life-saving treatments to the people who need them.
Here we focus on autologous therapies, such as chimeric antigen receptor T (CAR T) cells. However, many of the challenges also apply to allogeneic therapies, which offer the possibility to reduce cost of goods (1).
A truly collaborative approach
The United Kingdom’s network of Advanced Therapy Treatment Centres (ATTC) assembles members from various National Health Service hospitals and industry to develop solutions that accelerate the adoption of advanced therapies. The ATTCs are driving innovation, because challenges are tackled from multiple points of view – those of cell therapy manufacturers, clinicians, and the biotech industry – so that new solutions can be identified, developed, and tested. For example, members have discussed the need for a better way to ship the growing number of cryopreserved samples. World Courier and Cytiva, who are industry participants, have been working with stakeholders across the networks to understand and address logistics challenges.
Logistics challenges in today’s vein-to-vein workflows
For autologous therapies it is critical to capture all of the data throughout the vein-to-vein workflow to definitively show the chain of custody, chain of identity and chain of condition, ensuring that a patient’s therapy gets back to that same patient and is of the expected quality. However, this is not a simple task. The logistics element of the workflow involves at least three parties. At the end of the workflow, these parties include the clinical center responsible for receiving the therapy, the manufacturer responsible for supplying the correct, safe and efficacious treatment, and the logistics provider responsible for delivering the therapy to the clinical center. Some workflows also involve a contract manufacturing organization.
If the multitude of databases, data management systems, and dashboards from all logistics parties do not communicate, there is a risk of developing a therapy that cannot be administered.
How fragmented data impacts manufacturers
As cell and gene therapy manufacturers progress towards clinical therapies, they become more and more familiar with good manufacturing practices (GMP). But manufacturers might not be aware of distribution practices (GDP), which extends the quality assurance delivered by GMP in the manufacturing setting to logistics. GDP ensures that products are consistently stored, transported, and handled under the conditions required by the product’s specification. In this way, GDP bridges good manufacturing practices at the manufacturer and good clinical practices (GCP) at the clinical centers, stretching the quality pipeline across the vein-to-vein workflow.
Manufacturers looking to comply with GMP and GDP through a quality management system currently must pull data from many places in order to assemble documentation from the whole manufacturing and logistics process. Then, they must be confident of their cross-referencing. Compiling logistics data is especially challenging. For example, records are required to validate the sample handling process, which is usually a standard operating procedure (SOP) for loading and unloading the sample to and from the temperature-controlled transport container. This record is needed to trace the sample’s chain of custody linking it to a specific shipment container and to verify that the internal conditions were maintained throughout transit, as recorded by a data logger or smart wireless probe. Process delivery records are often paper-based in the clinical setting or held in separate record management systems.
Challenges multiply with number of patients
Even across the workflow for a single patient’s therapy, there is variation in how data is collected, formatted, and stored. Also, there is variation in the shipment monitoring data collected during transit and in the ability to access shipment data in real time. Often, this data is available only post-delivery, when it is too late to take action if there was an issue during transit. Variation is expected to become a bigger issue as the number of patient samples, clinical sites, and requirement for trained staff increases.
Clinical sites are particularly challenged as these treatments scale to reach more patients. They face administrative and process burdens to handle samples and shipments for multiple patients as well as temporarily store the shipment documentation, packing materials, and apheresis units or therapies. Logistics requirements vary with the sample, including different packing processes, temperatures, and urgency. SOPs for loading and unloading samples in shipping containers involve multiple steps and require staff with specialized training. Furthermore, clinical sites are frequently burdened with paper-based delivery records and rely on manufacturers or couriers to generate shipping labels.
Current cell therapy logistics workflows are not set up with scalability in mind. A small clinical trial might involve highly qualified people who are focused on just one or two treatments. These people can monitor the treatments closely and handle a lot of complexity. But it is not practical to expect that same level of oversight when treating thousands of patients a year. Also, a cell therapy manufacturer might have its own staff at the clinical site to receive the patient sample and monitor its administration. That level of control is not scalable.
A unified manufacturing and logistics solution
Today it is possible to digitally connect cell therapy manufacturing equipment using the cloud-based Chronicle software platform. This single digital space can manage the whole manufacturing process, through distribution, and then thawing at the clinical site for administration to the patient. The Chronicle software provides an additional layer looking across that entire process, automatically capturing data, events, and information from all sites that are using the software. In the manufacturing setting, Chronicle can create an electronic batch record of the vein-to-vein manufacturing process – starting with the raw materials collected in the clinical site and ending prior to infusion.
Chronicle has several features to address temperature-controlled logistics challenges of current workflows. For example, the software includes sample electronic SOPs that can guide operators through the loading process and digitally record the steps of the loading procedure. This feature can help to minimize the variation between operators and supports efforts to standardize logistics processes.
Other features are accessible via a specialized smart probe inside the shipping container. The internal conditions and GPS coordinates can be monitored remotely throughout the shipment to allow real-time tracking. The condition continuity data feeds automatically from the smart probe to a dashboard within Chronicle; if predetermined thresholds are exceeded or specific events occur, a specified contact is alerted. For example, an alert might be requested in the event that a temperature-controlled container is opened or placed on its side, both of which could lead to unacceptable rises in temperature. The software pulls all this data into a single combined logistics record. This unification makes chain of identity and chain of custody much more straightforward. Also, it gives users new insights, rather than massive streams of data, on a critical part of the workflow.
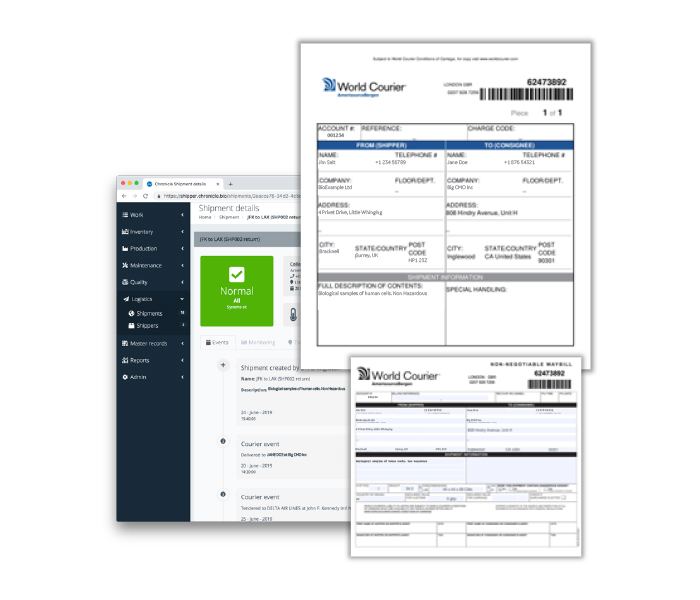
Additional benefits are provided through collaboration with World Courier™, a logistics platform developer that manages time- and temperature-critical shipments and focuses on advanced therapies. Chronicle users can book World Courier shipments directly in the software. All the shipping documentation can be printed to produce the packing list and the way bill, as well as the shipping label. When the shipment passes into World Courier’s hands, the events from the World Courier system are being pulled back into Chronicle, so that users have a record of the time the delivery driver collected it and all the places it was moved from there. All of that data is then combined with the SOPs and shipping documentation. Figure 1 shows some of the Chronicle features relevant to logistics.
(A)
(B)
(C)
Fig 1. Unified electronic record of all logistics records held in Chronicle, with enhanced features for World Courier customers. (A) eSOP directs and records process delivery and captures bar code information to verify chain of custody and identity; (B) condition continuity and location of shipper uploads to Chronicle dashboard from 3G monitoring probe; (C) World Courier shipment booking and logistics documentation available through Chronicle with record of courier handling events.
Collaboration drives innovation
The first collaboration between World Courier and Cytiva pairs World Courier’s global logistics services with Cytiva's FlexFactory platform for cell therapy manufacturing to support commercialization. Based on that successful venture, the companies joined forces again. In addition to gaining the logistics advantages of Chronicle software, users who choose World Courier for shipping can build a comprehensive record and more easily produce documents and labels. Through collaboration both inside and outside the ATTC network, both companies aim to continue bringing meaningful solutions to advanced therapy manufacturers, ultimately benefiting the patients they serve.
Learn more about the different Chronicle software options, which provide scalability from process development to clinical trials and commercialization.
Learn more about World Courier, including its Logistics by Design framework, which helps advanced therapy developers to develop commercially successful logistics platforms.
References
- Harrison, R. P. et al.Chimeric antigen receptor-T cell therapy manufacturing: modelling the effect of offshore production on aggregate cost of goods.Cytotherapy21, 224–233 (2019).