Your gene therapy candidate deserves to be a success. To fuel preclinical studies that use adeno-associated virus (AAV) for gene delivery, you need a ready supply of high-quality vector. Good laboratory practices (GLP) grade vector has the quality required for nonhuman primate studies and is a cost-effective option, because it avoids the infrastructure costs of good manufacturing practices (GMP) facilities. And the lead time to get GLP-grade vector could be much shorter.
You can get good laboratory practice (GLP)-grade vector from a new facility brought to you by Cytiva and the University of Massachusetts Medical School (UMMS). Here, you can access the combined benefits of Cytiva’s industry-leading development platforms and processing equipment plus UMMS’ professional and scientifically knowledgeable staff to help you get your research to the clinic faster.
The 299 m2 (3220 ft2) manufacturing facility at the UMMS, located in the Horae Gene Therapy Center, features GLP viral vector FlexFactory manufacturing platforms. These platforms integrate many of Cytiva’s systems for AAV production. Six professional staff manage the day-to-day running of the facility, which is now fully operational. The FlexFactory platform is part of the broader Cytiva Enterprise Solutions offering.
The facility produces AAV using SF9 cells at scales for investigational and translational gene therapy studies. These scales can be further developed into the GMP AAV production scale that is required for clinical and commercial gene therapy, through the Mass Biologics‑UMMS partnership (see Fig 1).
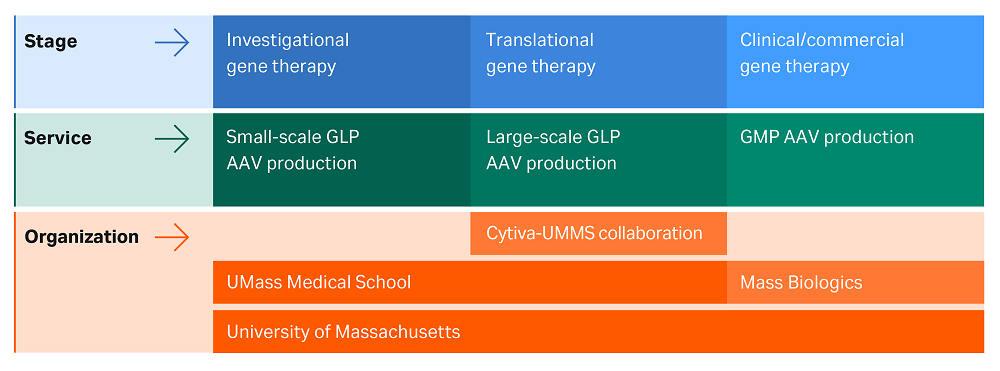
Fig 1. UMMS gene therapy ecosystem.
UMMS provides a world-renowned ecosystem for gene therapy, led by internationally recognized scientists who have pioneered research in gene therapy and have deep scientific knowledge.
“We are very excited by this collaboration with Cytiva and the addition of this large-scale AAV service to the Horae Gene Therapy Center. It now allows my team to support our collaborators in all of their preclinical AAV supply needs to help them bring their therapies to patients faster,” says Guangping Gao, PhD , Director of the UMMS Horae Gene Therapy Center and Viral Vector Core. He is also the president of the American Society for Gene & Cell Therapy (ASGCT). Dr. Gao played a key role in the discovery and characterization of a new family of AAV serotypes, which was instrumental in reviving the gene therapy field that is having a huge impact on many human diseases that are currently not treatable. Robert Kotin, PhD is one of the world’s top experts on the viral transport of gene therapies. A former NIH scientist and executive at Voyager, Dr. Kotin has played a sizable role in shaping the field.

Cytiva has a rich and long legacy of providing world-class, cutting-edge manufacturing platforms that can be tailored to project-specific requirements for viral vector production. In addition, Cytiva has expertise in taking companies from small scale to large scale.