Introduction
Adoptive cell transfer, in which a patient’s own immune defenses are boosted or rewired to kill cancer cells, is one of the most effective forms of personalized cancer care to ever reach patients. There are two types of adoptive cell transfer. In one type, immune cells called T cells are isolated, genetically modified, expanded, and returned to patients (e.g., CAR T cell therapy, TCR-modified T cell therapy). In the second type, the cells are expanded and infused back without genetic modification. The latter involves tumor-infiltrating lymphocytes or TILs – a naturally occurring, heterogeneous population of white blood cells that migrate into a tumor. TILs are known to be the most suitable immune cells to attack and destroy the cancer they homed in on. (For an introductory overview of the status and desirable evolution of TIL therapies, please refer to our previous article here.)
“Back in 1988, we published our first work showing that TILs isolated from patients with metastatic melanoma could be expanded in the lab and returned to the patient, where they mediated cancer regression,” stated Dr. Steven Rosenberg, chief of surgery at the U.S. National Cancer Institute, in a report by the American Association of Cancer Research (AACR) in 2018 (1). His decades-long research into TILs, along with multiple studies by other groups, have proven that TIL therapy shows significant, durable success in treating melanoma (2, 3). TIL therapy is now being explored for precision treatment of other types of solid cancers.
While it has been advanced over many years, the process of developing TIL therapies still suffers from certain challenges, particularly in the collection and processing of tumor samples used to extract TILs. This article attempts to lay bare some of these challenges and discuss how novel technological solutions can help overcome them.
Collecting tumor tissue for TIL therapies
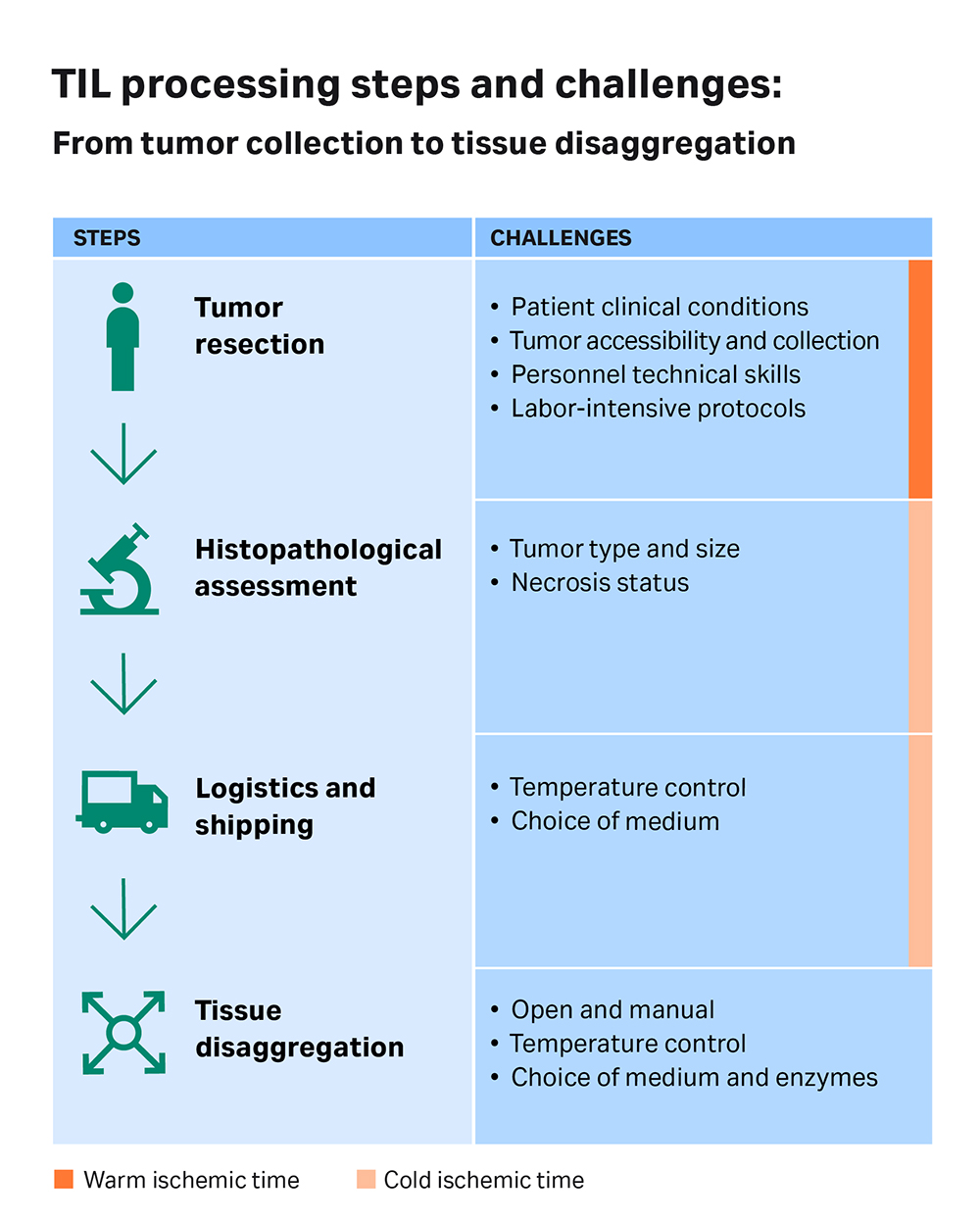
TILs are typically not present in high numbers within a tumor. Thus, they must be carefully extracted and handled to ensure that cell viability and yield is maintained. The first step in preparing TILs for therapeutics purposes is the collection of a sample of the solid tumor tissue. Dr. Sophie Papa, a Clinical Reader in immuno-oncology and Consultant Medical Oncologist at King’s College London, is a medical lead heavily involved in various clinical trials on TIL therapies. She says that “one of the greatest factors currently preventing patients from receiving TIL therapy is the difficulty of easily accessing and harvesting their tumors as a sample for processing.” The reason, she elaborates, is the labor-intensive protocols that require skilled personnel like surgeons and technicians who can expertly remove enough viable tissue.
After the tissue is taken in the operating room, it is sent to the pathology department. There, a pathologist decides how much of it can be allocated to research while keeping enough material for clinical and diagnostic needs. “Only pathologists can make this decision – they need to make sure that they do not harm the clinical output of the patient,” says Dr. Yehudit Cohen, Scientific Director of MIDGAM – the Israel National Biobank for Research, a governmental entity located at the Weizmann Institute for Science in Israel. Once the tissue is released by the pathologist, Dr. Cohen helps direct it to researchers for further research and processing.
Maintaining cell viability for higher yields
According to Dr. Cohen, the time between tissue resection and tissue submersion in the required medium (and the choice of medium itself) plays a significant role in cell viability and yield. “We measure this time length in two portions – the warm and the cold ischemic time,” explains Dr. Cohen. “We can only affect the cold ischemic time. The warm ischemic time [the time a tissue remains at the original body temperature after its blood supply has been cut off, but before it is cooled] depends on operation room procedures. For example, we can ask for the tissue to be delivered to us as soon as possible, but if the surgeon is still working and is not able to release the tissue, we can’t really change that.”
However, once the tissue is out of the operation room on ice, it must quickly arrive at the pathology department, where it is cut up and allocated for research and clinical purposes. There is some control over this ‘cold ischemic time’ to influence cell viability of the tissue given to a TIL therapy study. “The quality and viability of the tissue is dependent on how quickly it's cut up, how well its temperature is controlled, and how rapidly it's put into the medium that it's transported or disaggregated in,” Dr. Papa confirms. All of these steps introduce variability to the quality of the starting material, and, in the end, to the TIL therapy.
The viability of cells in the tissue sample is also affected by the donor's clinical condition. For instance, following chemo or radiotherapy procedures given to the patient, many cells may be necrotic, fibrotic and/or nonviable. Likewise, the type of tumor that is sampled can also impact the cell yield. “If you take colon or lung tumor tissues, you will probably have enough material to work with,” Dr. Cohen states. “But in contrast, most tumoral breast tissues are small – so, the potential of having enough tissue to work with is lower. Another example is pancreatic cancer. It’s very hard to access the tumor in a procedure like a biopsy – so collecting enough tissue is difficult, on top of the fact that TILs are present in low numbers.” Dr. Papa adds that some cancers have more lymphocytes infiltrating them than others, and these differences are inherently part of the biology of the disease.
At the National Center for Cancer Immune Therapy, University Hospital Herlev in Denmark, researchers have access to fresh tissue when isolating TILs for therapeutic purposes. “In our facility, we don't have to get chilled tissue from other parts of Denmark, because we usually have the patients in-house or in a nearby hospital,” explains Dr. Özcan Met, Associate Professor and Head of Cell Manufacturing at the center. Dr. Met’s team is able to expand TILs from resected tumor in more than 95% of patients with metastatic melanoma. He believes this is, at least, partly due to their rapid access to fresh tissue.
Fig 1. A schematic diagram showing the different steps in the process of developing a TIL therapy: from tumor collection to disaggregation. Critical aspects highlighted.
Improving manufacturing – automation, environmental control, and standardization
Experts like Drs. Papa, Cohen, and Met agree that there is a lot of room to improve the efficiency of the TIL therapy development process. Particularly, in the time period between physically removing tissue from a patient and transporting it to the laboratory – whether that's in the same building or on a different continent – several aspects can be optimized to enhance the quality and efficiency of the process.
Anything that involves the patient is hard to standardize. “Harvesting the tissue and treating the patient are two things that you can't standardize or automate at either end, but everything else in the middle we should be striving to automate and standardize as much as possible,” Dr. Papa suggests. Also, she believes that eliminating the need for a surgical procedure, where possible, would be helpful. Interventional radiology and biopsy-based techniques to obtain tissue are much less complex than surgery and would speed up the process. According to Dr. Papa, technologies that could enable rapid tissue disaggregation, maybe directly at the bedside or in the operating theater, would improve the quality of the harvest.
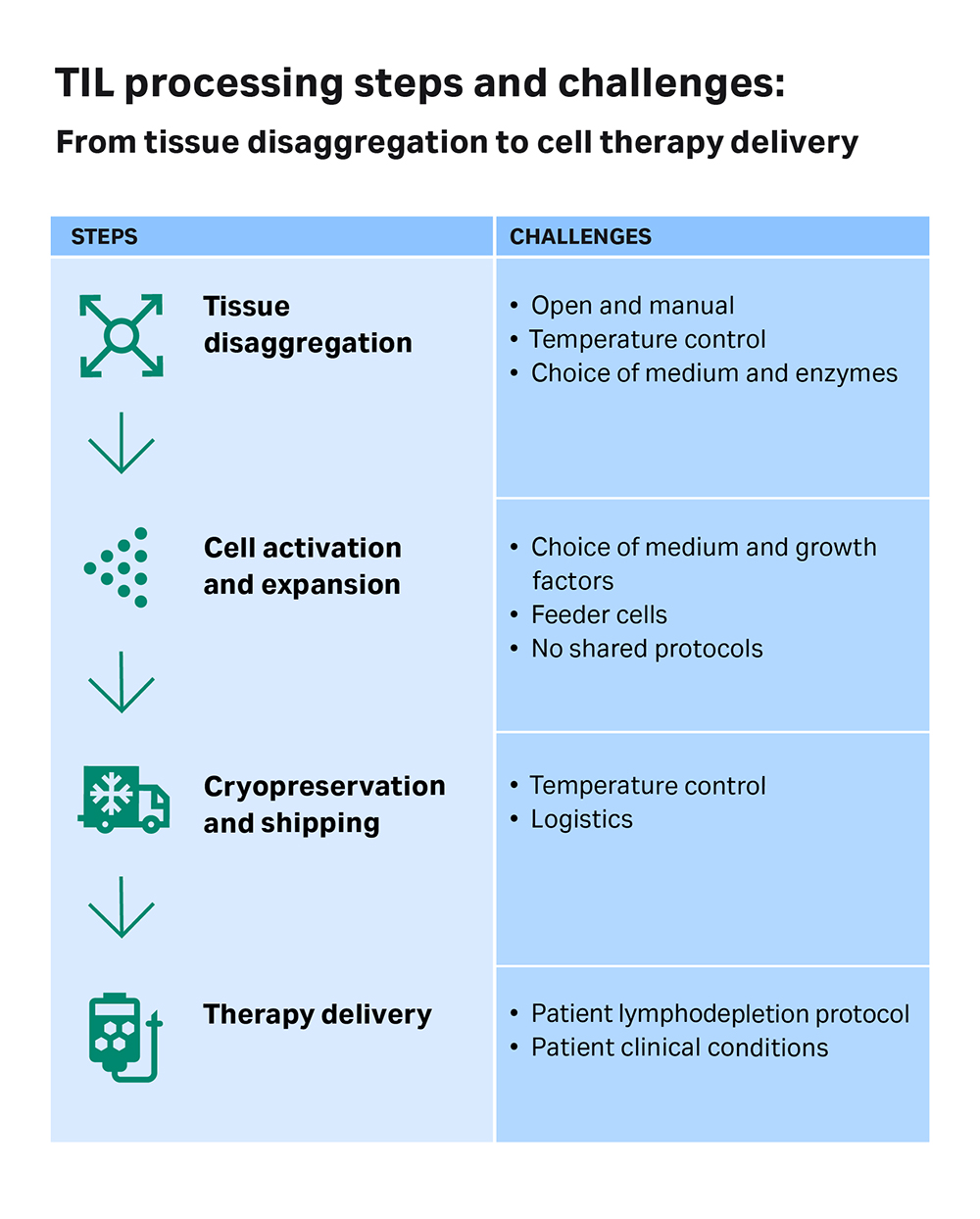
Once the samples are received in the lab, they are taken into a sterile environment where the tumor is disaggregated and the cells grown and expanded in medium supplemented with growth factors, cytokines (e.g., high dose of IL-2), and supporting feeder cells. Throughout this manufacturing process, there are multiple quality checkpoints for the cells, but much of the process is not standardized or temperature controlled.
According to Dr. Papa, there are many different protocols using various reagents and supporting cells for different periods of culture. “Traditionally, and in some current circumstances, a lot of this is done in a manual, open manner,” she notes. Such open systems lack effective monitoring of the process temperature, which can result in low yields, contamination, and inconsistencies between samples. Moreover, the tumors used to extract TILs may contain contaminants from the beginning.
Fig 2. A schematic diagram showing the different steps in the process of developing a TIL therapy: from tumor disaggregation to therapy delivery. Critical aspects highlighted.
Right now, one of the biggest hurdles in trials of TIL therapies is the lack of standardization of protocols, whether it’s in sample collection or processing. “If you've got multiple different centers that are recruiting to your trial, the more standardized things are, the more you can compare results, like whether patients respond to treatments or not, and be confident about the quality of the cellular product,” Dr. Papa says. Her recommendation to reduce variability is to make the upstream process of getting tumor samples for the therapy manufacturer as simple, automated, standardized, and regulated as possible.
Dr. Met agrees that there should be more standardization and optimization in the upstream steps. He explains that TIL therapy is a very specialist form of therapy, and different countries have different regulations for manufacture. “For instance, in Denmark, we can use allogeneic feeder cells for the expansion of the TILs, but this is not allowed in some other countries and they have to use autologous feeder cells,” Dr. Met adds, pointing out that changing manufacturing protocols requires extensive validation, which is both costly and time-consuming. He believes there’s more leeway to optimize sample processing before expansion, and industry could help in this regard with collaborations and new technological innovations.
“It would be really helpful to have more sharing of information relevant to sample processing, so that there can be more standardization across protocols,” concludes Dr. Papa. “That way, we can draw conclusions more confidently across trials about the efficacy and feasibility of the therapy.”
References
- Olsen K. TIL therapy explained by Steven Rosenberg, MD, PhD. AACR. November 2018. Accessed July 6, 2020.
- Rosenberg SA, Packard BS, Aebersold PM, et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med. 1988;319(25):1676‐1680. doi: 0.1056/NEJM198812223192527
- Met Ö, Jensen KM, Chamberlain CA, Donia M, Svane IM. Principles of adoptive T cell therapy in cancer. Semin Immunopathol. 2019;41:49-58. doi: 10.1007/s00281-018-0703-z