A lateral flow immunoassay, or immunochromatographic assay, is a rapid, convenient test that uses antibodies (or sometimes antigens) to detect specific macromolecules, such as antigens, drug substances, or other proteins.
One of the first steps in lateral flow rapid test development is the selection of the “right” materials, especially the nitrocellulose (NC) membrane. The NC is the heart of the lateral flow test, where the target molecule is bound and where the results are displayed.
A successful lateral flow immunoassay depends on a series of reactions. When selecting materials for immunoassay development, membrane properties and reagent properties must be considered together, as their interactions determine the outcome of test development.
When selecting a membrane, you must consider how the various materials and properties will interact with your reagents and sample to meet your test goals for sensitivity, specificity and test duration. Factors that will affect the interactions between your sample and the membrane include:
- Viscosity of the sample liquid
- Compatibility of the test reagents with the membrane surfactant
- Kinetic properties of the binding reagents
Membrane specification: using capillary flow time is key
Capillary flow time is the time a liquid (usually water) needs to migrate along a defined distance (usually 4 cm) parallel to the surface of the membrane, using a membrane strip of defined width (e.g. 1 cm). For details, see Figure 1. The shorter the time, the “faster” the membrane.
With increasing distance from the bottom of the membrane, the residence time of the migrating liquid at any point of the membrane also increases. The further a capture reagent is immobilized from the bottom of the membrane, the more time the capture reagent will have to find and bind its target (Fig 2). A test developer may be able to use this fact to improve the test performance.

Fig 1. Basic set-up of membrane capillary flow time measurement. Strip width is 1 cm, strip length 4.5 cm, with triangular marks at 4 cm strip length. The time the liquid (water) needs to reach these marks is taken and documented. Water volume is 100 µl.
Sample viscosity affects immunoassay speed
Comparing the membrane capillary flow time specification (for water) with the serum capillary flow time in Figure 2 demonstrates how the viscosity of the sample liquid can affect the test duration. The greater the viscosity, the longer the capillary flow time., which results in longer test duration. The routine way to deal with a long test duration is to select a membrane with a faster capillary flow. This will have to be balanced against the kinetic properties of the capture reagents. The capillary flow time specification can provide an indication of whether the membrane is “fast” or “slow,” but you will have to test it with your sample to determine if it can meet your test requirements.
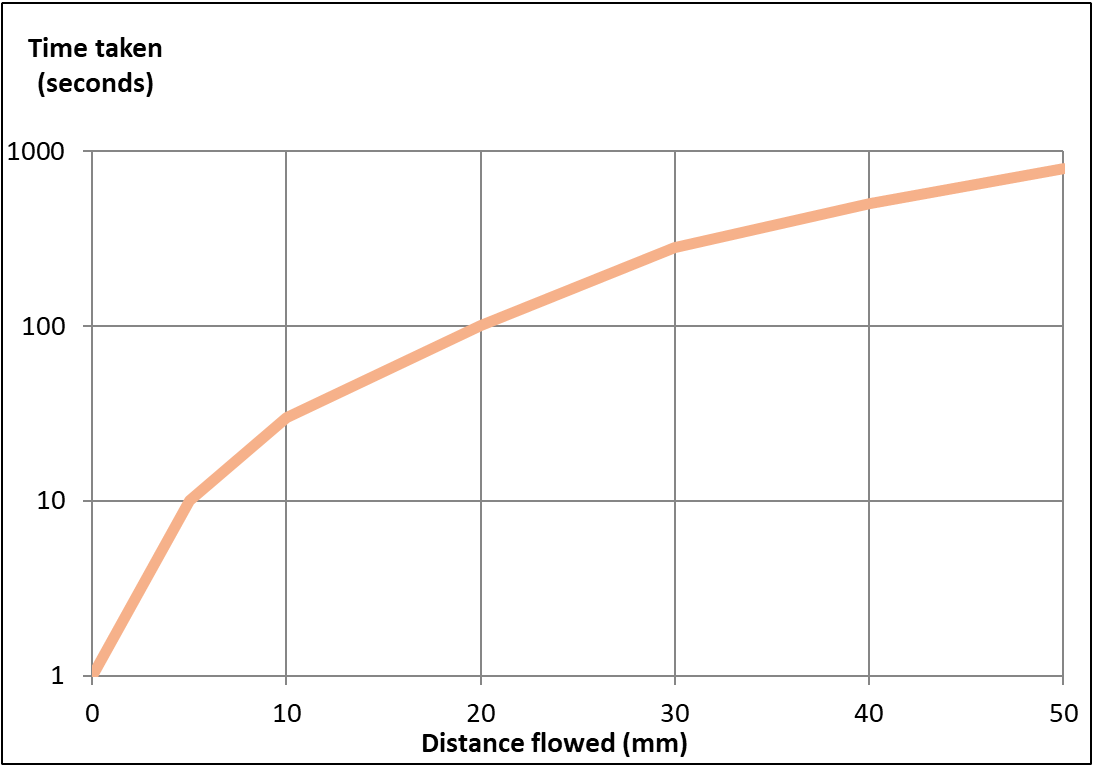
Fig 2. Capillary flow time of human serum on an AE99 lateral flow membrane (Capillary flow specification with water: 120–160 seconds). The capillary flow time of human serum was determined at different distances from the bottom of the membrane using the method outlined in Figure 1. The flow rate of the serum decreases with increasing distance from the bottom of the membrane strip, so flow rate is not useful as a membrane specification.
Alignment of membrane specification and immunoassay properties
Capillary flow time specification is generally given in a range. While most samples of a membrane will fall in the middle of the range, some may fall at either edge. It is of vital importance to evaluate whether your sample and test reagents are compatible with a specific membrane grade, even if it was manufactured at one of the edges of the specification. Possible issues are indicated in Figure 3.
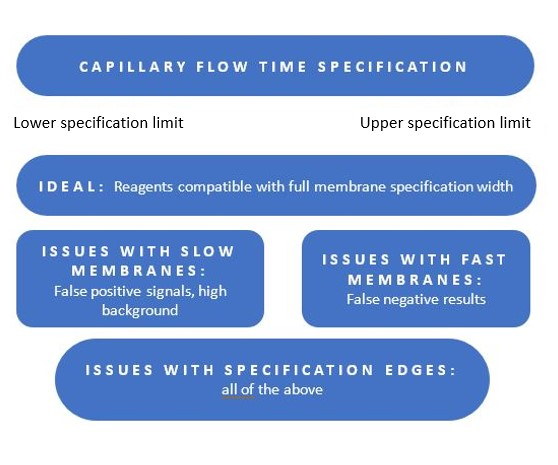
Fig 3. Possible issues with respect to lateral flow test requirements and membrane capillary flow time.
Membrane surfactants and protein binding: good news/bad news
Every nitrocellulose membrane on the market contains a surfactant, usually an anionic surfactant. The exact nature of the surfactant is proprietary for every membrane manufacturer, but the surfactant functions are always the same. First, it makes the membrane hydrophilic. Second, and perhaps even more important, it helps proteins bind to the membrane (Fig 4).
The good news: The surfactant partially denatures proteins dispensed onto the membrane in a buffer and helps them bind to NC fibers.
The bad news: The surfactant denatures proteins. This can potentially destroy the antigen binding sites of some monoclonal antibodies completely, making these reagents unusable in a lateral flow test.
From our experience, about 2–3 % of clones are affected by this problem, and others are partially impaired by this process. In these cases, it makes sense to evaluate different membranes with different surfactants for their performance with your specific molecule and reagents.
Cytiva has developed three membrane families (FFHP, FFHP Plus, and Immunopore). Each of these membrane families contains a specific surfactant at a defined concentration, which is identical for all the membrane grades in a family.
A summary of the properties of the different Cytiva membranes can be found at the end of this article.
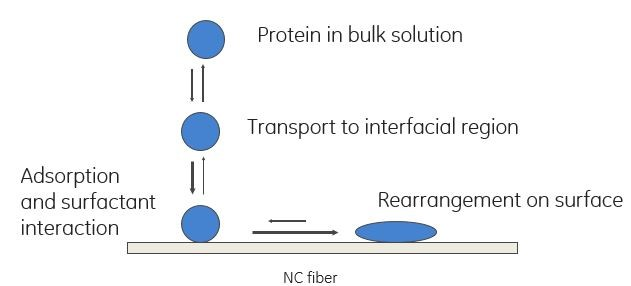
Fig 4. Schematic representation of the process of protein binding to an NC membrane.
Binding kinetics of capture reagents and membrane compatibility
Whether a specific capture reagent, e.g. a monoclonal antibody, provides the sensitivity and specificity required in a lateral flow rapid test depends not only on the membrane used, and the surfactant incorporated in the membrane; it also depends on the kinetic properties of the capture reagents.
The affinity of an antibody is determined by its on-rate, the rate at which an antigen binding site grabs its target, and its off-rate, the rate at which the bound antigen is released again from the binding site. Antibodies with identical affinities for a specific target may have very different on- and off-rates, as described in Figure 5.
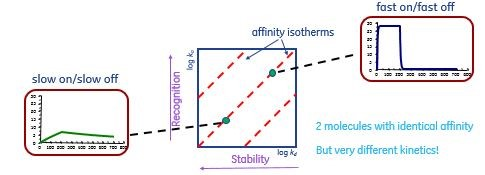
Fig 5. Kinetic properties of different monoclonal antibodies with identical affinities for a specific antigen.The data were obtained using surface plasmon resonance with Cytiva Biacore equipment. Antibodies with slow on- and off- rates (left) can easily have the same affinity (center) as antibodies with fast on- and off- rates (right).
The residence time of an antigen at the test line of a lateral flow test is usually very short, always lower than a minute, sometimes only a few seconds. Therefore, antibodies with high on-rates are required for lateral flow rapid tests.
A test developer should evaluate all available capture proteins for their target antigen in the test system itself, or, more favorably, by surface plasmon resonance (SPR) prior to antibody selection. The latter approach enables the developer to exclude clones with obviously unfavorable binding or releasing kinetic properties.
If a test must be developed with a specific pair of capture and detector reagents simply because there are no alternatives available, there might be a conflict between the sensitivity goals and the goals for test duration that have been defined before the development was started. Capture reagents with slow on-rates may result in a low sensitivity test on a fast membrane, although only this membrane provides the developer with the “right” test duration time. If this happens, it sometimes helps to look for membranes with different surfactants and/or surfactant concentrations, but the ultimate outcome might be that either the original goals of the test development have to be adjusted to what is achievable with this set of reagents, or new reagents may have to be developed.
Membrane selection: finding the right balance
Membranes for lateral flow assays are available at different capillary flow times and different surfactant contents. The flow time is influenced by the viscosity of the sample liquid. The slower the flow time, the longer it will take to clear the membrane from the background of the sample liquid, and the more time the test needs until completion. On the other hand, a slow flow time allows for a longer interaction time of the targets to be detected at the test line(s), which can enhance the sensitivity and specificity of the test.
Membrane properties and reagent properties are not independent of each other: their interactions will determine the outcome of a test development. For example, it may be that the reagents used in a specific test development enable the use of a low viscosity sample on a fast membrane, or they may require the use of a slow membrane even with a high viscosity sample. The surfactant used on the membrane may affect the binding ability of your sample.
A summary of the base properties of Cytiva lateral flow membranes and initial recommendations for which membrane may be used for which type of sample liquid can be found in Table 1 below. If you are currently developing a lateral flow assay device or looking to re-validate a new membrane, request a sample of our FFHP nitrocellulose backed membranes for improved assay consistency and reproducibility.
For Samples or to contact a Specialist
RELATED CONTENT
- Component selection in lateral flow assay development (blog)
- Membrane selection for lateral flow immunoassays (article)
- Custom lateral flow assay projects (article)
- Lateral flow assay troubleshooting guide & how to switch diagnostic membranes (article)
- Infographic: Considerations for lateral flow membrane selection
- Infographic: 10 Top Tips for lateral flow assay development
- Infographic: Navigating flow-through assays
RELATED PRODUCTS