The fine balance of photon collection in image capture
The art of capturing biomolecular images is, at its heart, about harvesting photons. Collecting more photons from a sample results in sharper images and detection of faint protein bands which otherwise could have been missed. More photons also leads to a greater signal-to-noise (S/N) ratio and increased quantitation confidence.
The present generation of ECL reagents for chemiluminescence-based detection, such as ECL Prime, uses the enzymatic horseradish peroxidase (HRP) luminol reaction to deliver a stable, high signal. In fluorescence-based detection, CyDye fluorophores are the industry standard and now span across the entire visible range, with Amersham CyDye 700 and 800 near infrared (NIR)-labeled antibodies recently taking center stage.
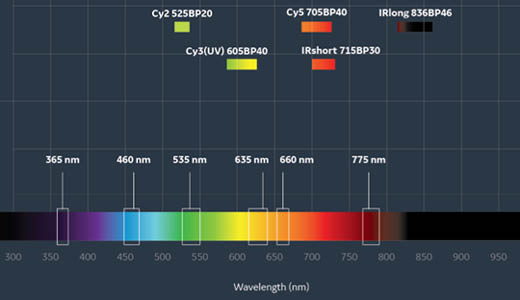
Fig 1. There is a wide range of fluorescence excitation and emission options available on the Amersham ImageQuant 800.
Modern imagers have light sources, emission filters, and lenses designed to maximize detection of emitted sample photons. If more detected photons are better, when in the imaging process should we stop collecting photons and how can we decide the best exposure time? Image capture is usually allowed to continue to obtain maximum signal, with the limiting factor being saturation of bands of interest.
Grayscale images in .TIF format, composed exclusively of gray shades or levels, are used for quantitation in applications like Western blotting. In a 16-bit .TIF file generated by the imager, there are 65 535 gray levels. When surplus photons are collected, the maximum image pixel value remains at 65 535, regardless of the exposure time, and results in saturation. Yet, we might still want to collect more photons to see details in the images and detect weak bands. So, how can we overcome this challenge?
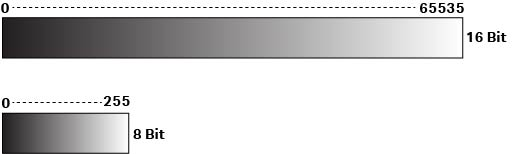
Fig 2. Grayscale .TIF files are composed of different levels of gray shades. 16-bit files have a higher number of gray scale levels than 8-bit files.
SNOW detection for image optimization
The Amersham ImageQuant 800 biomolecular imager introduces a novel capture mode called SNOW (named for Signal-to-Noise Optimization Watch) which allows for the collection of photons for longer exposure times without saturation. The technology is based on recording each image after an appropriate time to avoid saturation, and then averaging all recorded images into a final image. Image details are more visible and linear dynamic range is extended, avoiding saturation.
SNOW imaging mode also allows users to view image quality improvement in real time and auto-stops when the best image with the highest signal-to-noise ratio has been achieved. This capability eliminates the trial and error of image capturing and removes the uncertainty that comes with selecting one image from many for analysis. Simply start the automatic SNOW imaging mode on the Amersham ImageQuant 800 system and come back to view the final optimized image.

Fig 3. Amersham ImageQuant 800 CCD imager with SNOW imaging mode.
In this article, users of the Amersham ImageQuant 800 CCD systems can find information on how to leverage SNOW detection to capture the best images. The SNOW detection mode can be used with both ECL and fluorescence detection of proteins on a Western blot and for any binning setting, permitting image capture of even the most demanding samples for which high resolution is critical.
Two-fold S/N improvement in chemiluminescence detection
Amersham ECL Prime is a highly sensitive chemiluminescent detection reagent characterized by extremely stable signal emission. Combined with SNOW detection mode, this reagent enables longer exposure times and higher signal-to-noise ratios, resulting in better band resolution.
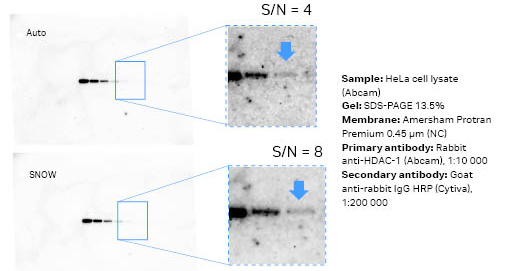
Fig 4. Images of the same membrane from auto mode (7 min, first) versus SNOW mode (49 min, second) using ECL Prime. The signal from the main band is approximately 30 000 counts for both images. The limit-of-detection band had twice the S/N ratio for the SNOW capture.
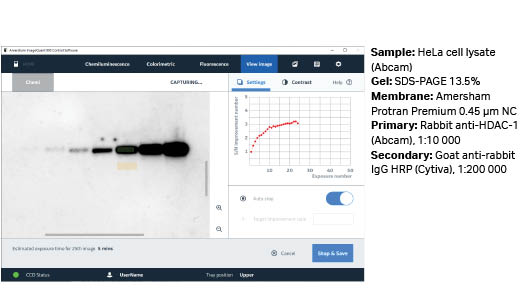
Fig 5. Example of signal-to-noise improvement during SNOW capture using ECL Prime.
Over three-fold S/N improvement in fluorescence detection
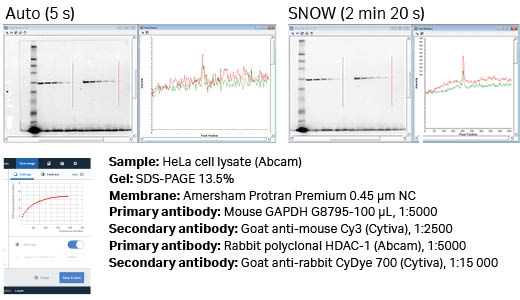
Fig 6. Auto versus SNOW with Cy3 detection of GAPDH using Amersham ECL Plex Cy3. The S/N of the weakest detectable band was improved by 3.5 times, from hard to observe (auto) to easy to quantitate (SNOW).
Visibly distinguish and detect weak bands in NIR region
Amersham CyDye 700 secondary antibodies (Fig 8) are labeled with NIR fluorophores that emit light at wavelengths of 700 nm.
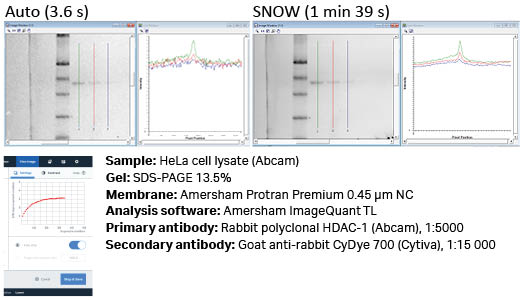
Fig 7. It is possible to detect additional bands with SNOW which could not be detected using the standard auto-capture settings, as is shown here with IR-short detection of HDAC1. There is clear improvement in noise, as can be seen in the line profiles.
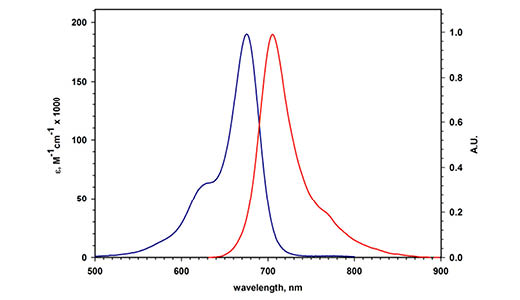
Fig 8. Excitation and emission spectra of Amersham CyDye 700 secondary antibodies.
Achieve exceptional linearity even while detecting weak signals
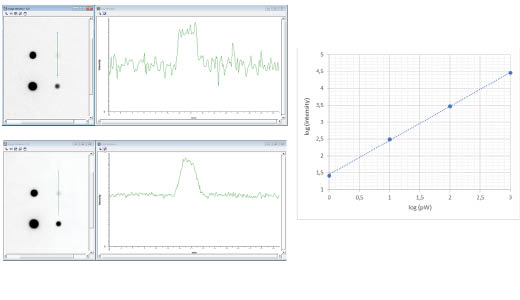
Fig 9. Auto (1 s) versus SNOW (27 s) of a calibrated light source which emits 1 pW, 10 pW, 100 pW, and 1000 pW. Both images show excellent linearity (k = 1.01) across the dynamic range and the SNOW image exhibits almost four times lower noise levels compared to the auto image.
Noise reduction to detect weak protein signals

Fig 10. Amersham host cell protein (HCP) DIGE membrane with CHO lysate (50 μg) and Cy3 detection of K1 control protein (1:200 dilution). The auto image was captured in 1.5 s and the SNOW image in 2 min 29 s, both with 1×1 binning. The noise level is markedly improved with SNOW, which allows detection of weak bands with greater confidence.
Reduce noise and detect strong and weak signals without saturation
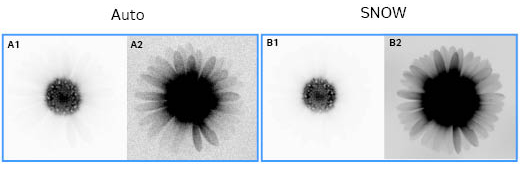
Fig 11. An auto IR-short (0.1 s) image (A1, A2) compared to a SNOW captured (17.7 s) image (B1, B2). The contrast of the A1 and B1 images was set to view the strong signal of the central flowers, and the contrast of the A2 and B2 images was set to view the white florets. The extended dynamic range allows both the weak and strong signals to be captured in the same image with SNOW.
High resolution imaging across the entire spectrum
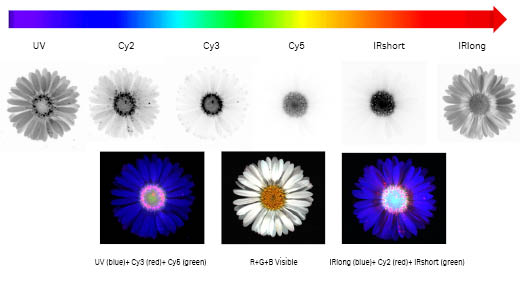
Fig 12. The Amersham ImageQuant 800 imager allows high resolution imaging across the entire visible spectrum, from UV to infrared. The daisy Bellis Perennis contains multiple yellow disc florets and white ray florets. The open disc florets display a characteristic fluorescence emission observed with UV, Cy2, and Cy3 LED-filter settings compared to the budding florets in the center. In the fake color overlays, these open florets appear as pink dots, approximately 0.5 mm wide. The Amersham ImageQuant 800 1×1 binning allows such sub-mm details to be clearly resolved.
Conclusion
When combined with detection reagents that offer long signal stability in chemiluminescence, such as Amersham ECL Prime, the SNOW detection mode can be invaluable in detecting weak signals without saturation. In fluorescence detection mode, this novel setting also helps achieve high sensitivity and high resolution without saturation. SNOW detection on the Amersham ImageQuant 800 imager increases signal-to-noise ratio and improves image quality for accurate quantitation in biomolecular research.