GST-tagged protein purification
What is GST-tagged protein purification?
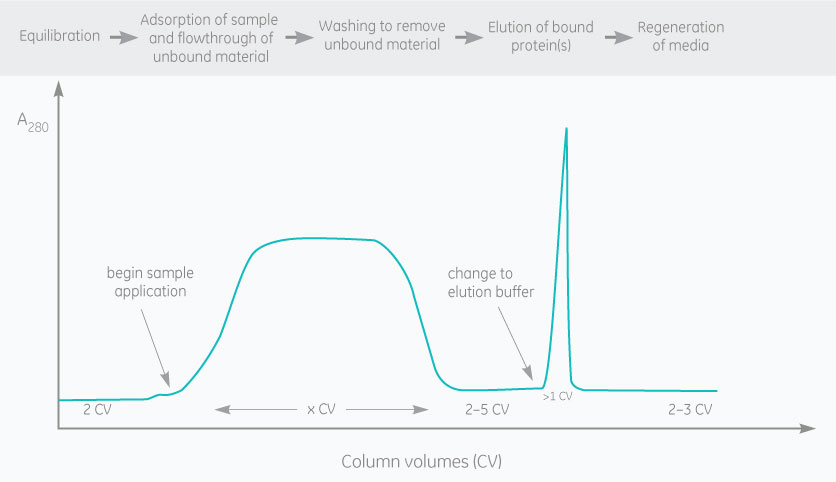
Recombinant proteins that have Glutathione S-transferase (GST) tags are purified using affinity chromatography. GST binds strongly and specifically to chromatography resins coupled with glutathione.
Proteins are eluted under mild conditions that preserve protein function, Protein purities higher than 90% can be obtained after a single purification on Glutathione Sepharose. Because GST is large, it often needs to be removed from the target protein before use.
How does GST-tagged protein purification work?
Purification of GST-tagged proteins is based on the affinity of GST to the glutathione ligand coupled to a matrix. Glutathione Sepharose resins are often used for purification. The binding of a GST-tagged protein to the ligand is reversible, and the protein can be eluted by adding reduced glutathione to the elution buffer.
Optional removal of the GST tag can be performed on the column or after elution.
When should I use GST-tagged protein purification?
GST-tagged protein purification can be used as the only purification step for applications that do not require proteins with the highest purity.
When extremely high purity is needed, this technique can be used as the first (capture) step in a multistep purification procedure.
For example, size exclusion chromatography (gel filtration) can be used after GST-tagged purification to remove co-purified proteins.