His-tagged protein purification
What is histidine-tagged protein purification?
Histidine-tagged protein purification uses affinity chromatography to capture recombinant proteins with 4–10 histidine residues. Histidine-tagged proteins can be purified from a wide range of expression systems under native or denaturing conditions. The main drawback is that the technique often requires optimization to minimize nonspecific binding of host cell proteins. Protein binding capacity of resins is typically 10–40 mg/mL. Purity up to 95% can be achieved with optimized conditions.
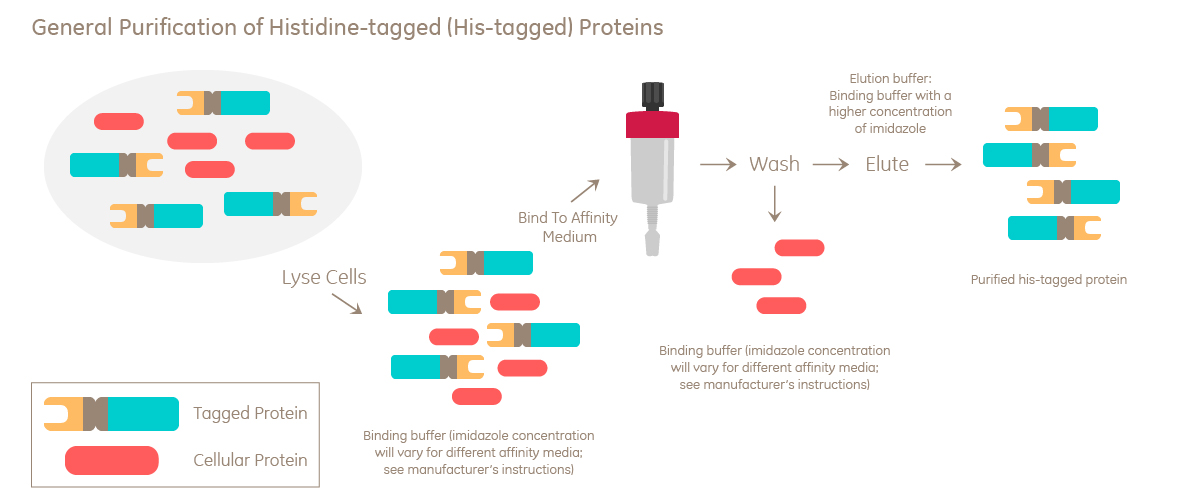
How does histidine-tagged protein purification work?
Histidine-tagged proteins are commonly purified using Immobilized Metal Affinity Chromatography (IMAC). IMAC is based on the interaction between amino acid residues and divalent metal ions immobilized on resins. Histidine interacts most strongly, and histidine-tagged proteins have extra high affinity because of the multiple histidine residues.
The chemical imidazole is used to elute histidine-tagged proteins. A lower concentration can also be used to wash resins prior to elution.
When should I use histidine-tagged protein purification?
Histidine-tagged protein purification can be used as the only purification step for applications that do not require proteins with extremely high purity. When the highest purity is needed, this technique can be used as the first (capture) step in a multistep purification procedure. It is also suitable for purifying dual-tagged proteins with a histidine-tag at one end and a different tag at the other end.
Histidine-tagged protein purification can be used to purify proteins expressed as inclusion bodies.
How can I remove a histidine-tag?
The histidine-tag is small, so it usually does not have to be removed.
Remove the tag if:- it interferes with the function of the target protein
- the target protein needs to be in a native state (e.g., for structural studies)
Cleave the tag using a protease that recognizes the protease recognition sequence in the target protein. Some recombinant proteases are histidine-tagged, so they can be easily removed after a second pass over the resin used to purify the protein of interest.