Biologics developers and manufacturers demand an accurate and reliable assay for host cell protein (HCP) quantitation. Let’s take a close look at why analytical scientists turn to the ELISA, and how it fits into process development.
What are host cell proteins?
Biological drugs (or biologics) are manufactured by living systems such as microorganisms, and plant and animal cells. Cell lines, like Chinese hamster ovary (CHO), can be engineered to work as cellular factories to produce biologics in addition to their own biological molecules.
Host cell proteins (HCPs) are effectively biological by-products of these cellular factories. They are one of the main impurities in harvested cell culture fluid (HCCF), and tend to be released when cells die or are damaged, rather than by active secretion. HCPs can influence a drug’s efficacy and toxicity, and have long-term immunogenicity, if not removed during manufacture.
Indeed, worldwide pharmacopeia, including the US and EU, include regulatory guidelines for detecting HCPs and purifying the biological drug. Let’s take a closer look at how analytical scientists measure host cell proteins, and what makes the enzyme-linked immunosorbent assay (ELISA) the gold standard.
HCPs and process development
Part of process development for any new biologic is detecting the unique combination of HCPs and confirming that the purification processes reduce them to a safe level, typically in the nanograms of HCP per milligrams of drug range.
As you might imagine, there’s a multitude of factors that can affect HCPs. Each host cell line will have its own unique signature of expressed proteins. Even within the same cell line, any differences in upstream culture conditions—temperature, acidity, nutrient supply, and so on—can influence the exact combination and abundance of HCPs.
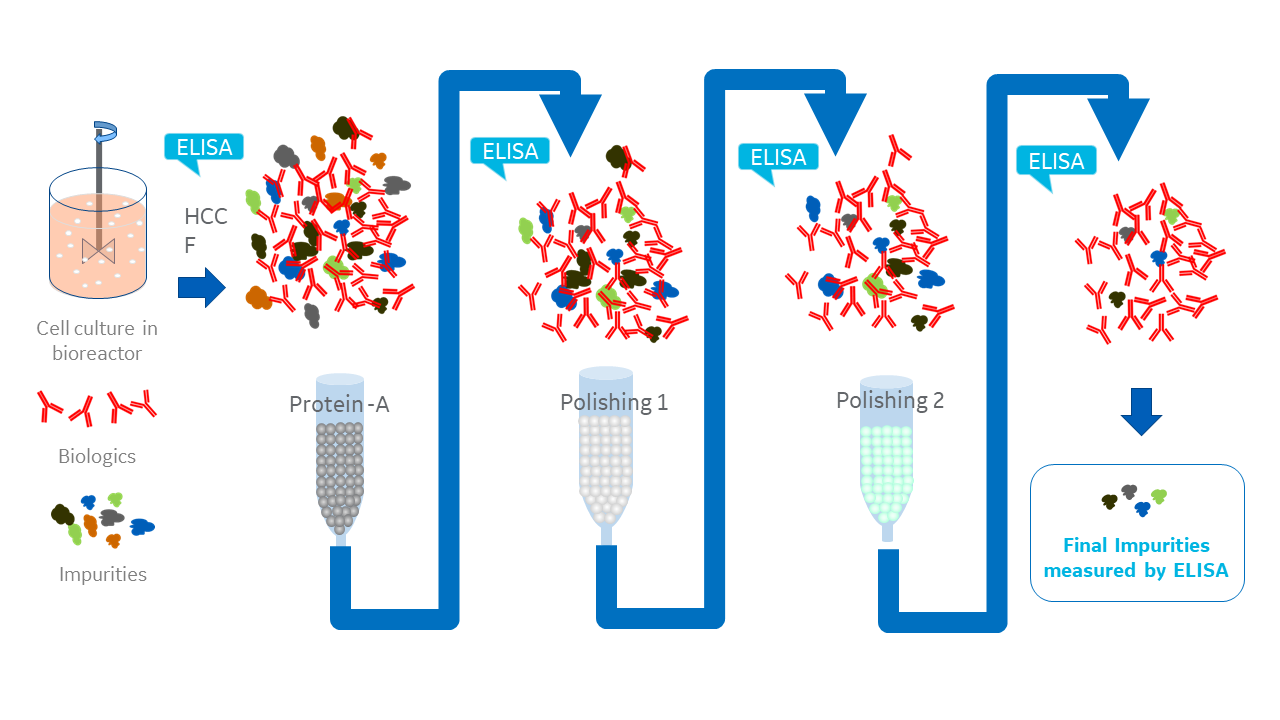
The result is that any change you make upstream will probably have downstream effects on HCPs. So, as part of process development, you’ll likely need to adjust and optimize downstream purification steps at various points, for example, after Protein A affinity and ion exchange chromatography (Fig 1).
As the intention here is to reduce HCPs, a reliable and accurate assay is essential to check the effect of each adjustment and purification step: the sandwich ELISA. This assay’s sensitivity and selectivity make it well-suited for detecting the complex mixture of an HCP population. They are also easy to perform and automate, and can be high throughput.
Fig 1. ELISAs are essential at every step of biologics process development
How are ELISAs used to measure HCPs in biologics production?
There are 3 potential modes of use for any assay in HCP analysis: detection, quantitation, and identification (USP 1132). ELISAs make detection and quantitation straightforward, while orthogonal assays like mass spectrometry, can deal with identification, when needed.
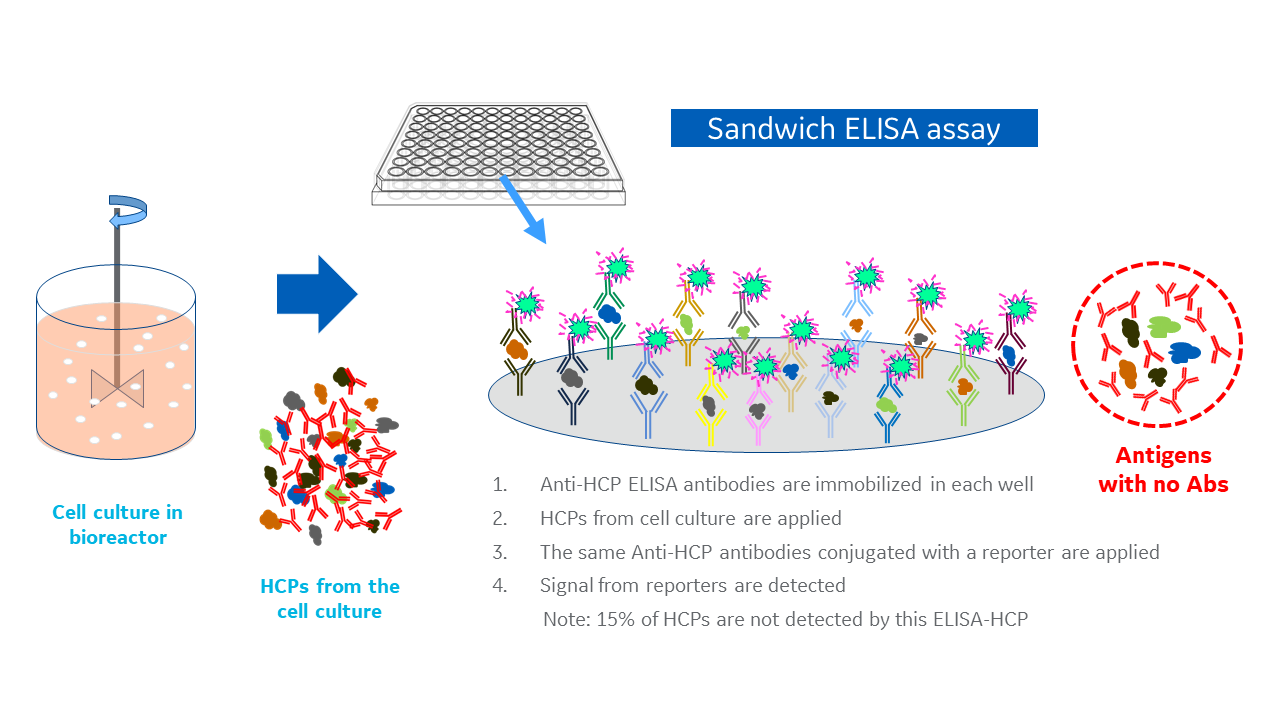
ELISAs rely on the affinity and selectivity of antibodies for their target antigen, in this case the HCP population. Detecting the presence and abundance of HCPs follows a sandwich ELISA protocol (Fig 2), which would usually use a single antibody per assay. But, given the complexity of an HCP population rather than just one, you need a multitude of polyclonal antibodies against the complex population of HCP antigens in one multi-analyte assay.
The process for raising these antibodies generally involves:
- Culturing the null cell line with a mock or empty expression cassette.
- Immunizing an appropriate animal host, for example a rabbit, goat, or chicken, with the HCPs.
- Extracting the antibodies raised by the host and checking coverage of antigens by 2D gel electrophoresis/Western blot.
Bio-pharmaceutical companies might use commercially available generic kits for these assays, and/or develop process- or platform-specific approaches (USP 1132 and Ph. Eur. 2.06.34), dependent on need, stage in development, and cost.
Fig 2. A typical sandwich ELISA for host cell proteins
The different approaches to ELISA
Generic kits are commercial products developed to work across a broad range of similar expression hosts, for example CHO cells. These are a straightforward option, but vendors often won’t disclose detailed information on assay preparation. Also, the cell line used might not mimic the one you use or cover the same range of HCPs.
These kits are useful for general characterization, however, and make a good starting point for biologics manufacturers during process development in early phases of clinical research.
Process-specific assays are the most specialized type and built around a specific product. Often developed by specialist providers, these account for the upstream and downstream processes specific to a product. In theory, this could provide the most complete quantitation of HCPs possible by ELISA for that product, but requires a considerable time and cost investment.
Platform assays can be developed in-house to suit a specific expression platform, such as a variant of a cell line, reducing the need for generic and process-specific assays. The strength of this approach is in broad coverage and high sensitivity, making them applicable to multiple products manufactured using the same cell line and similar processes.
The US Pharmacopeia Chapter 1132 suggests that, once developed, platform assays can be validated for subsequent products, and take the place of generic assays through all stages of development, reducing the per product investment.
Detect and measure HCPs with the HCPQuant CHO generic ELISA kit
Not so complete coverage?
Despite being the benchmark assay, ELISAs can’t detect everything. It’s unlikely you would get 100% coverage of all HCPs using the raised antibodies, even with the process-specific approach.
Some HCPs won’t be immunogenic enough to trigger a response in the animal host, or will only trigger a low response. Any antibodies with low affinity for the antigen might not be detectable by ELISA at all!
So, to use ELISAs for HCP quantitation, regulatory bodies ask that each assay be validated. In many protocols, this involves running 2D gel electrophoresis/Western blot and comparing the anti-HCP antibodies detection to an in-gel total protein stain.
This type of coverage assay enables you to estimate a percentage coverage, and can direct you towards using a more specific ELISA or orthogonal assays, like mass spectrometry, that could fill the gap.
The increasing significance of checking coverage is shown by recent updates to US and EU pharmacopeia in 2016 and 2017 respectively (USP 1132 and Ph. Eur. 2.06.34). Japanese pharmacopeia on the same subject is under preparation.
Although these updates are just guidelines and best practices, most analytical scientists now consider coverage assays an essential part of product development.
In the next post, I’ll review coverage assays, and provide tips for improving their accuracy and speed. If you have any questions or would like support with optimizing your host cell protein analyses, contact our Scientific Support team, or your local Cytiva representative.
Learn more about Cytiva's end-to-end solution for HCP analysis