Towards commercial lentiviral vector production
As gene transfer vehicles, lentiviruses exhibit many desirable properties, such as high transduction efficiencies, ability to infect both dividing and nondividing cells, and stable integration into the host cell genome. These properties make them well-suited for in vivo and ex vivo gene and cell therapies (1). However, cost-effective manufacturing of lentiviral vectors (LV) at commercial scales has proven difficult and remains a pressing issue for the marketing of therapies that depend on their application.
The most established approach for lentiviral vector production is transient expression of vector components in adherent HEK 293 (or derivative) cell lines by a three or four plasmid co-transfection (2). However, large-scale production is severely limited in adherent systems. Because of these limitations, commercial manufacturing of LV is moving to cells adapted to serum-free growth and suspension systems that are much more amenable to scale-up. Further, stable-inducible producer cell lines could eventually replace transient systems for LV production, because this approach circumvents the costs and supply chain limitations of high-quality or cGMP-grade plasmid DNA and transfection reagents, as well as the inherent variability of transfection efficiencies across production lots (3, 4).
To control production costs and meet demand, stirred-tank bioreactors have been considered in place of adherent cultures for lentiviral vector production. The suspension culture approach can enable true scale-up of the process as opposed to scale-out strategies that are usually associated with adherent systems. In parallel, there is a need to identify media and supplements to maximize LV production in suspension-adapted cells. Also, it is important to develop simple and robust manufacturing workflows that are straightforward to scale up to the desired production batch sizes.
The workflow described here is a scalable upstream process that evaluated several commercially available options meeting these criteria. Although LVs are traditionally produced in adherent cells that rely on animal serum supplementation, all processes in this study were performed using suspension-adapted HEK 293 cells and animal-derived component-free (ADCF), chemically defined media and reagents. A stable-inducible LV producer cell line, HEK293SF-LVP-CMVGFPq-92 (clone 92), was used as a model producer cell in this study (5). Clone 92, which encodes a third generation conditionally self-inactivating LV carrying the transgene for the green fluorescent protein (LV-GFP), was adapted to serum-free suspension culture and is stable without the need for selection.
In this study, lentiviral vector production was achieved in short and simple batch processes. Production was successfully scaled in a linear manner from 5 L to 28 L in single-use, stirred-tank bioreactors.
See detailed Materials and methods.
Results and discussion
Medium selection and small-scale lipid supplementation
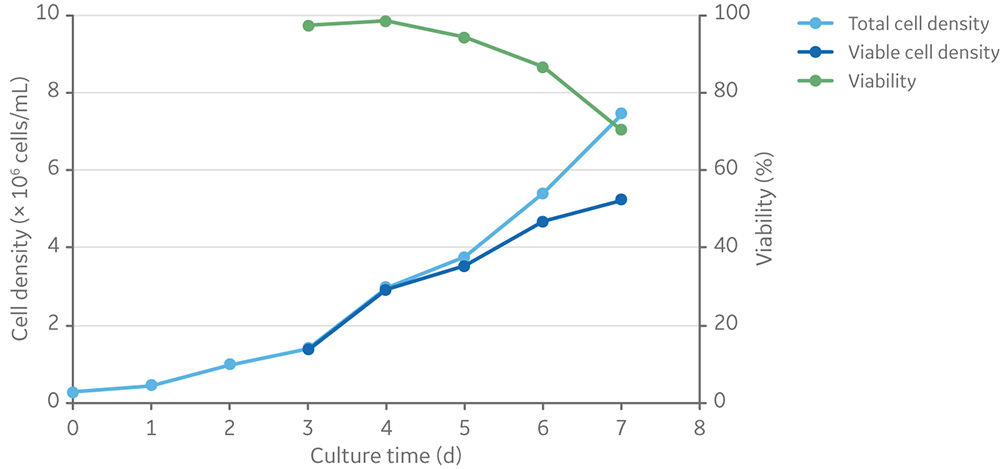
HyClone HyCell TransFx-H was selected as the culture medium because it is ADCF, fully chemically defined, and supports the growth of clone 92 producer cells in suspension when supplemented with L-glutamine and a surfactant. After four days of batch culture, clone 92 cells reached a viable cell density of 3 × 106 cells/mL in HyCell TransFx-H medium without a detectable loss of viability (Fig 1).
Fig 1. Viable cell density and viability of clone 92 cells batch cultured for 7 days in TransFx-H medium with L-glutamine and a surfactant.
A baseline batch process for LV production from clone 92 cells includes a pre-induction expansion of cells to 1–2 × 106 cells/mL and induction of vector production by addition of cumate and doxycycline (which activate transcription of rev and vsv-g genes). This process yielded on average 6.7 × 106 TU/mL of LV-GFP, which is harvested at peak titers three days post-induction (Fig 2).
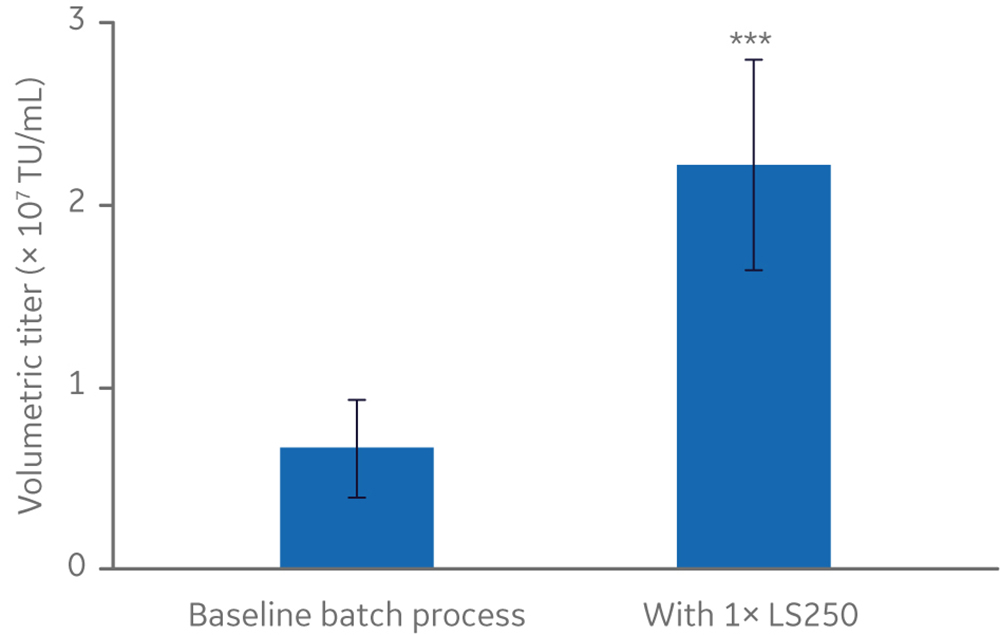
Manufacturing vectors is a major contributor to the cost of manufacturing cell and gene therapies that rely on them (2). Improvements in vector production, such as increased vector yield per batch, can reduce manufacturing costs and increase the availability of therapies. Considering that LV acquires its lipid envelope from the plasma membrane of host cells, lipid supplementation of culture medium was explored as a strategy to improve vector yield (6). Screening of several cell culture additives in small-scale cell culture identified the HyClone LS250 lipid supplement, a proprietary ADCF, chemically defined formulation of fatty acids and cholesterol, as significantly improving volumetric titer by three-fold or more, relative to the baseline batch process in non-supplemented medium (Fig 2).
Fig 2. Effect of HyClone LS250 supplementation on functional lentivirus titer from a stable producer cell line. n=5. All cultures ≤ 20 mL. TU/mL = transducing units/milliliter. Statistical analysis: T-test, two-tailed, paired. *** p-value = 0.002.
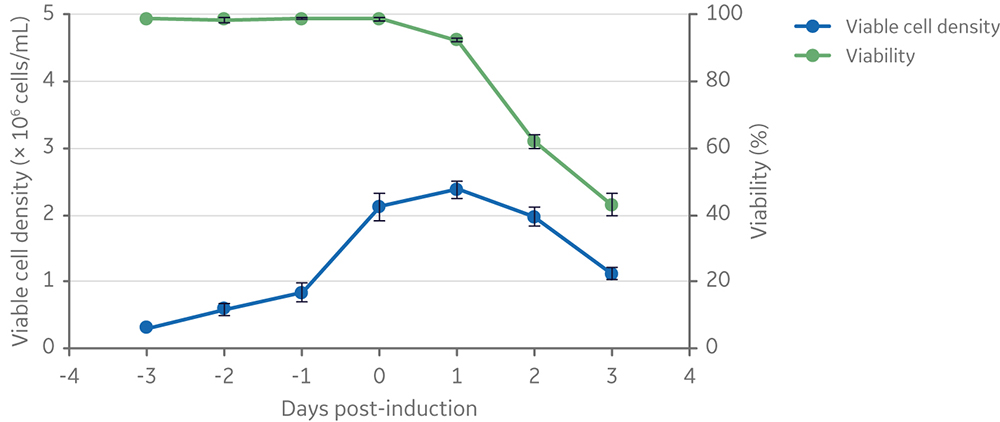
The LS250 lipid supplement is a 250× concentrated solution, which was diluted to 1× final concentration prior to cell inoculation at the start of a production cycle. Viable cell density and viability of clone 92 cells before and after induction of vector production are summarized in Figure 3. This figure shows a typical pattern of cell expansion prior to induction followed by a gradual loss of viability due to the expression of cytotoxic components (i.e., the viral protease and the glycoprotein VSV-G) of the lentiviral vector.
Fig 3. Viable cell density and viability of cells from inoculation to vector harvesting at 3 days post-induction. Clone 92 cells were cultured in 20 mL of TransFx-H medium supplemented with L-glutamine, a surfactant, and LS250. n=3.
In small-scale shake flask cultures, supplementation with LS250 yielded on average 2.2 x 107 TU/mL of LV-GFP. Additional experiments showed that supplementing LS250 at higher concentrations or delaying its addition until or after induction has neutral or negative effects on vector titer; we did not further explore the mechanism behind these negative effects (data not shown).
Scale-up to 5 L and 28 L cultures in Xcellerex XDR single-use bioreactors
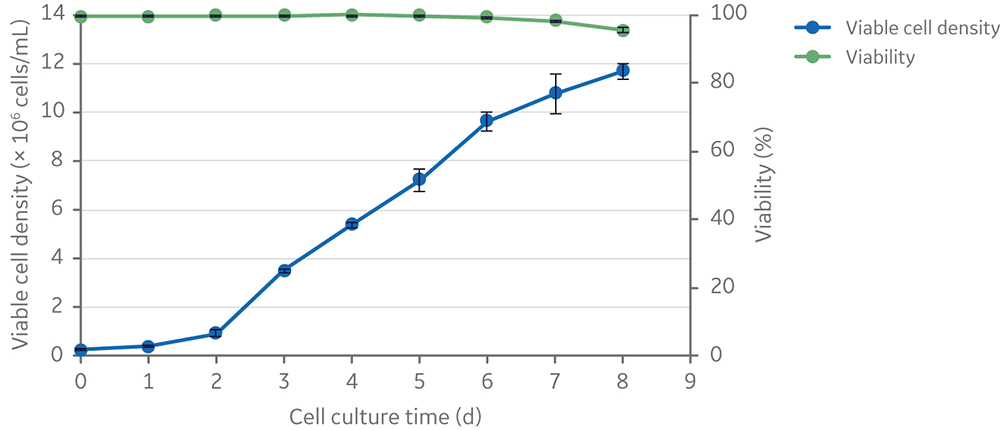
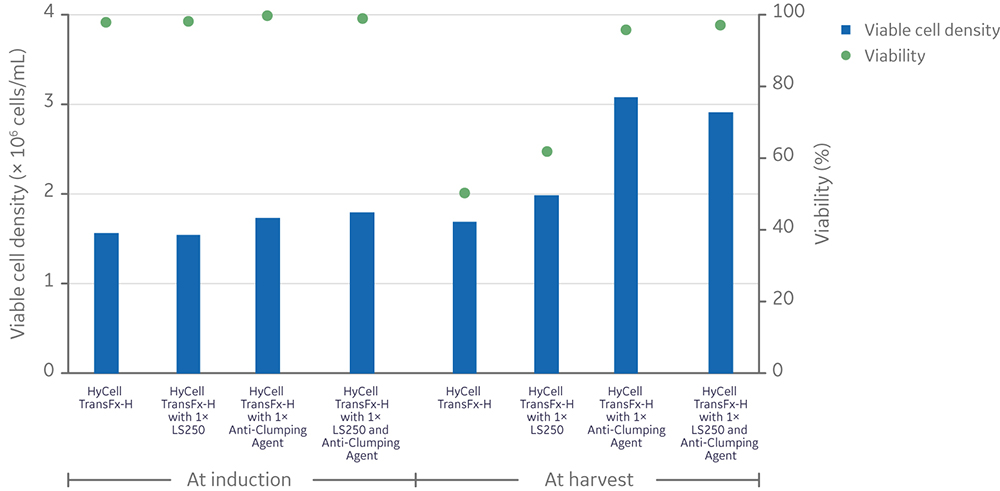
The addition of an ADCF, chemically defined anti-clumping agent was tested to enable scale-up of clone 92 cell culture and prevent excessive cell aggregation and gradual loss of cell viability observed in the initial scale-up attempts. Addition of anti-clumping agent (at 1x concentration) to small-scale (20 mL) shake flask batch cultures resulted in expansion of clone 92 cells to ~ 107 cells/mL with only a slight drop in viability (from 100% to 95%) after 8 days in culture; however, cells grew at a slower rate beyond 4 × 106 cells/mL (Fig 4).
Fig 4. Viable cell density and viability of clone 92 cells cultured in 20 mL of TransFx-H medium supplemented with L-glutamine, surfactant, LS250, and an anti-clumping agent.
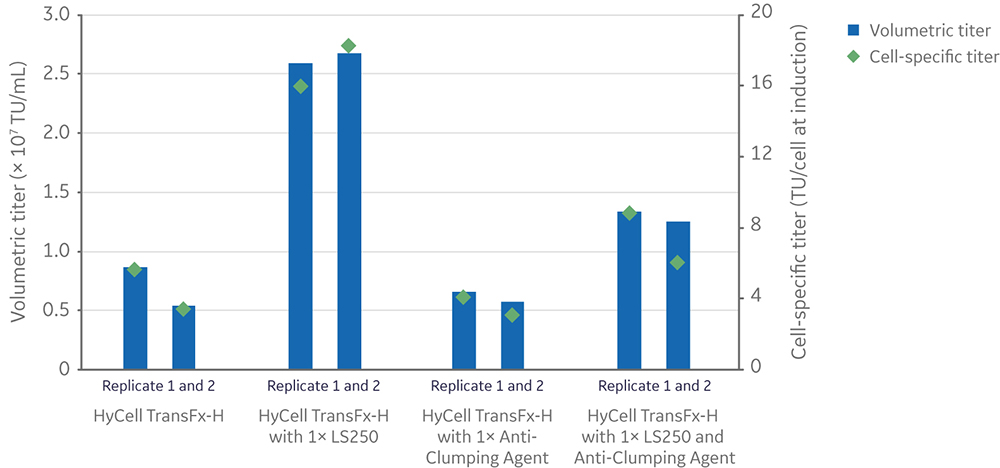
Following the usual small-scale production workflow (where cultures of 1–2 × 106 cells/mL are induced and incubated for three days), the anti-clumping agent reduced the positive effects of LS250 lipid supplementation on volumetric (TU/mL) and cell-specific (TU/cell at induction) vector titers (Fig 5).
Fig 5. Effect of anti-clumping agent on volumetric and cell-specific LV titers in 20 mL cultures with or without LS250 supplementation.
Regardless of LS250 supplementation, the anti-clumping agent had a significantly positive impact on the viability of cells (n=4, p-value < 0.05) measured at the conclusion of vector production, while having no significant effect on the total (live and dead) cell density (n=4, p-value > 0.05) (Fig 6).
Fig 6. Effect of anti-clumping agent on viable cell density and viability in 20 mL cultures with or without LS250 supplementation prior to (left) and after (right) LV production. Each data point is an average of two replicates. Statistical analysis of viability was performed by a t-test between conditions with and without anti-clumping agent (n=4).
Although the anti-clumping agent was mostly incompatible with LS250 supplementation for improving lentiviral vector production, it was included in the scale-up strategy because it did not decrease yields below the baseline process. Another consideration was the greater viability of cells at the conclusion of vector production compared to cultures without anti-clumping agent. This could lead to less dead cell host cell DNA and protein in the cell culture supernatant, which is harvested for downstream lentiviral vector purification and concentration.
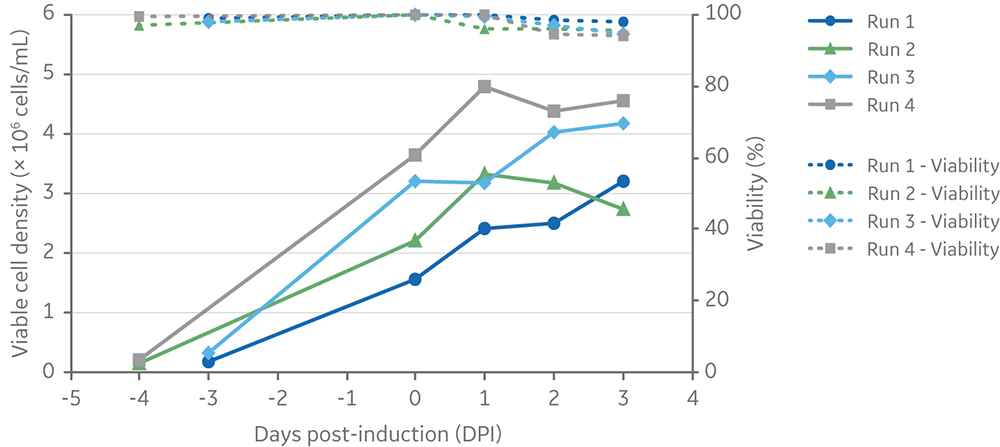
The Xcellerex XDR platform of single-use bioreactors, which is scalable from 4.5 L to 2000 L cultures, was selected to demonstrate scale-up of the process using ADCF, chemically defined media and reagents. Four runs were performed at 5 L scale in the XDR-10 stirred-tank bioreactors using four different induction cell densities. Cultures were induced at three or four days post-inoculation. Three days after induction of LV production, cell viabilities were > 90% for all runs (Fig 7). Final viable cell density ranged from 2.7 × 106 to 4.5 × 106 cells/mL.
Fig 7. Viable cell density and viability of cells from inoculation (at different cell densities) to harvesting of vectors at 3 days post-induction. Clone 92 cells were cultured in an XDR-10 bioreactor in 5 L of TransFx-H medium supplemented with L-glutamine, a surfactant, LS250, and an anti-clumping agent.
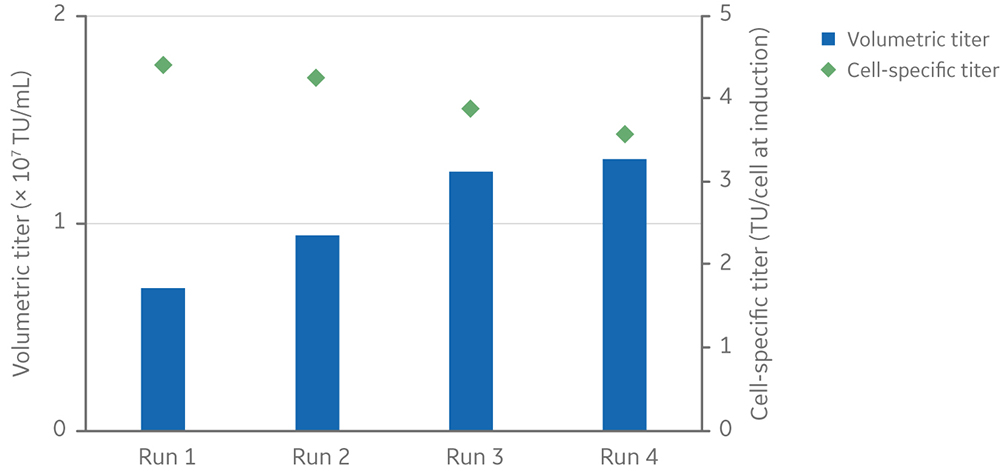
Volumetric LV yield ranged from 6.9 x 106 to 1.3 x 107 TU/mL of culture, and the average cell-specific LV yield was 4 TU/cell at induction (Fig 8).
Fig 8. Viable cell density and viability of cells from inoculation (at different cell densities) to harvesting of vectors at 3 days post-induction. Clone 92 cells were cultured in an XDR-10 bioreactor in 5 L of TransFx-H medium supplemented with L-glutamine, a surfactant, LS250, and an anti-clumping agent. Volumetric and cell-specific LV titers of four 5 L cultures in an Xcellerex XDR-10 stirred-tank bioreactor. Cultures were induced at different viable cell densities.
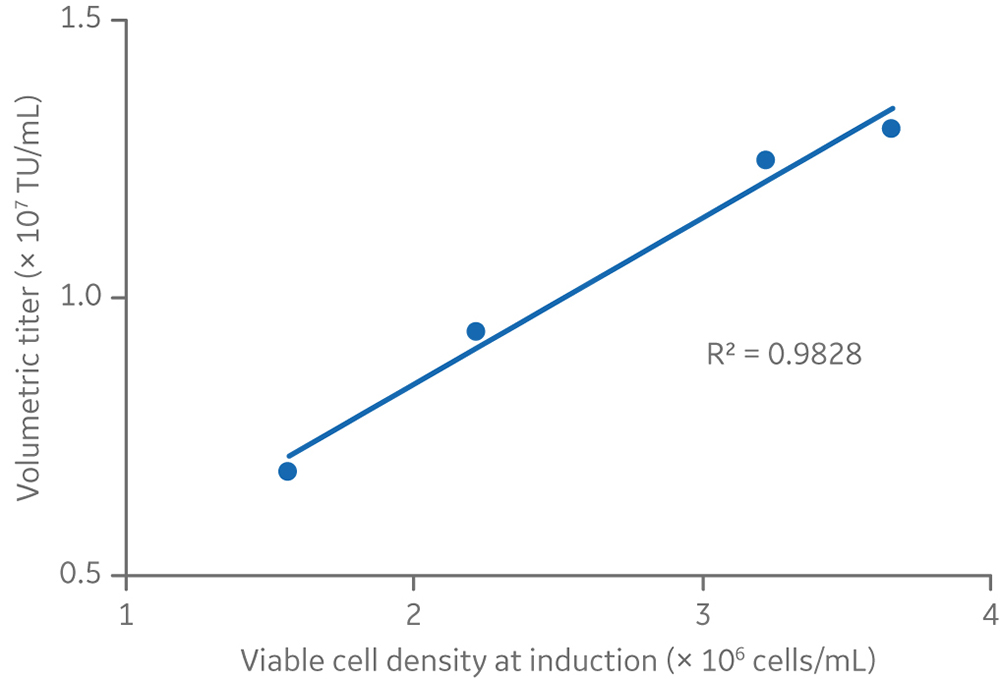
There was a linear relationship between viable cell density at induction and volumetric lentiviral vector yield (Fig 9). However, in small-scale experiments where cells were induced at densities > 4 × 106 cells/mL, both volumetric and cell-specific yields were lower (data not shown). These results reflect the requirement of cells to be in an exponential growth phase and below 4 × 106 cells/mL for best productivity, as can be seen by the cell growth kinetics presented in Figure 4.
Fig 9. The relationship between viable cell density and volumetric titers at the 5 L production volume.
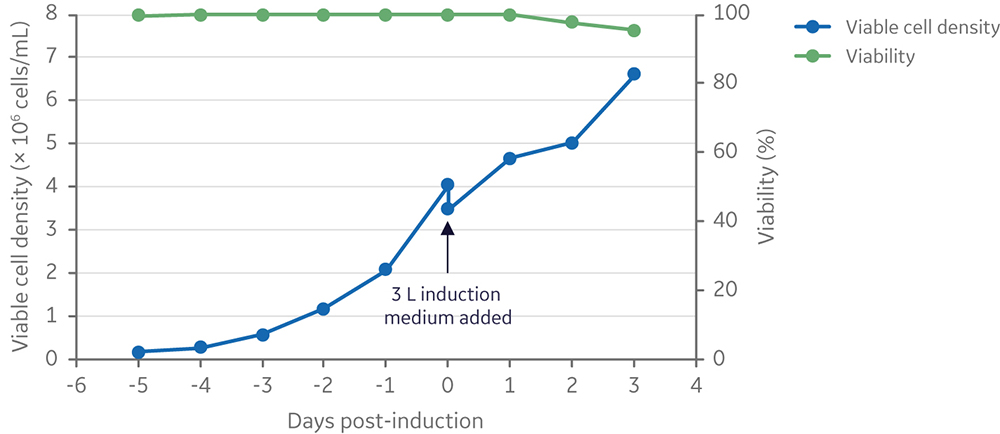
Lentiviral vector production was further scaled to a 25 L starting culture volume in a 50 L Xcellerex bioreactor, XDR-50. Cells were cultured for five days and then induced by addition of inducers diluted in 3 L of medium, for a final production volume of 28 L (Fig 10). The viable cell density at induction was 3.5 × 106 cells/mL. This was in line with run 4 at the 5 L scale (and close to the induction viable cell density of run 3), to ensure a comparable vector yield (≥ 1 × 107 TU/mL).
Fig 10. Viable cell density and viability of cells from inoculation to harvesting of vector 3 days post-induction. Clone 92 cells were cultured in the XDR-50 system in 25 L TransFx-H medium supplemented with L-glutamine, a surfactant, LS250, and an anti-clumping agent.
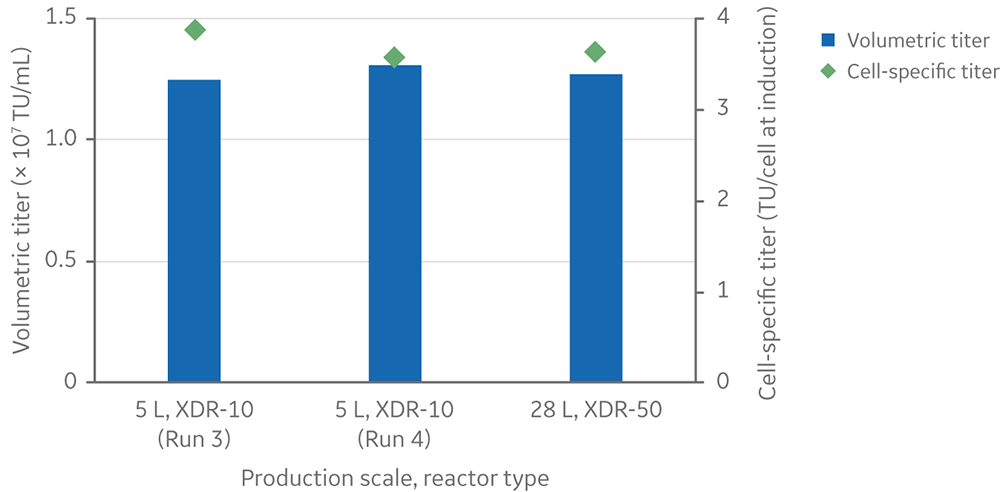
For the 28 L process, the volumetric LV yield was 1.3 × 107 TU/mL of culture, and the cell-specific LV yield was 3.6 TU/cell at induction (Fig 11). The volumetric and specific productivity closely matched what was observed at 5 L scale when using similar viable cell density at induction, confirming a process that scaled up in a linear manner.
Fig 11. Volumetric and cell-specific LV titers at the 28 L production scale in the Xcellerex XDR-50 single-use bioreactor. Runs 3 and 4 at the 5 L scale in XDR-10 were included for comparison.
Conclusion
Here we show a scalable lentiviral vector manufacturing process using a suspension-adapted LV producer cell line. Media and reagents are animal-derived component-free and chemically defined. The process scales in a linear manner from 5 L to 28 L in single-use bioreactors and yields ≥ 1010 TU/L of LV-GFP. This workflow can be adapted for engineered producer cell lines encoding clinically relevant LV vectors at commercial scales.
Learn more about our tools and techniques for producing lentivirus and other viral vectors.
Acknowledgements
All work was performed in collaboration with CCRM through funding from FedDev Ontario and Cytiva at the Centre for Advanced Therapeutic Cell Technologies (CATCT), Toronto, Ontario, Canada. The reporting and interpretation of the research findings are the responsibility of the author(s).
Ordering information
| Product | Product code |
| HyClone HyCell TransFx-H liquid medium | SH30939.02 |
| HyClone LS250 lipid supplement | SH30555.01 |
| Single-use Xcellerex stirred-tank bioreactors | 29054858; 29054859 |
| XDR-10 Pro Bag | 888-2-0396-C |
| XDR-50 Pro Bag | 888-0086-C-2 |
References
- Milone, M. C. and O'Doherty, U. Clinical use of lentiviral vectors.Leukemia 32, 1529–1541 (2018).
- Merten, O. W. et al.Production of lentiviral vectors.Mol. Ther. Methods Clin. Dev. 3, 16017 (2016).
- McCarron, A. et al.Challenges of up-scaling lentivirus production and processing.J. Biotechnol.240, 23–30 (2016).
- Sanber K. S. et al.Construction of stable packaging cell lines for clinical lentiviral vector production.Sci. Rep.5, 9021 (2015).
- Manceur, A. P. et al.Scalable lentiviral vector production using stable HEK293SF producer cell lines. Hum. Gene Ther. Methods 28, 330–339 (2017).
- Rodrigues, A. et al.Production of Retroviral and Lentiviral Gene Therapy Vectors: Challenges in the Manufacturing of Lipid Enveloped Virus in Viral Gene Therapy (Xu, K. ed.), InTechOpen (2011).