Adjusting downstream processes to increasing upstream titers
Single-use technologies offer the flexibility needed to meet the demands of multiproduct facilities. The capital investment is lower, and substantial time savings can be made when eliminating cleaning and cleaning validation procedures. While widely adopted in upstream production processes, what is required to move single-use technologies into downstream manufacturing scale? This article discusses strategies for meeting the ever-increasing upstream titers and volumes to prevent downstream purification from becoming a bottleneck.
Taking single-use chromatography to manufacturing scale
As the facility design often has limited expansion possibilities, other strategies are required for downstream processes to keep up with upstream production. These strategies can include easy scale-up of chromatography steps, higher-capacity chromatography resins and systems, and shorter process times. In contrast to upstream processes, where the disposable essentially constitutes a container, the disposable chromatography column also includes the resin, to which a large part of its cost can be attributed. A more intensive recycling of the packed column would therefore improve process economy, making adoption of single-use equipment also in downstream processes more feasible. Obtaining process approval will also be easier as regulatory authorities gain familiarity with the safety profiles of these devices.
ADRESSING THE SCALABILITY CHALLENGE IN SINGLE-USE COLUMNS
Historically, prepacked single-use columns have been limited in scale, partly due to handling and transportation challenges and partly due to challenges related to cost. At the larger scales typically found in manufacturing, the cost and time benefits of removing column packing and cleaning were outweighed by the limited number of cycles that could be performed with these columns. However, during the last few years, prepacked columns with sufficient column diameters for commercial scale have been introduced. ReadyToProcess columns, for example, are available with inner diameters of up to 600 mm, for design of disposable purification processes from bioreactor high titer harvests of up to 2000 L.
The development of high-capacity chromatography resins has further improved opportunities for start to finish disposable commercial processes. MabSelect PrismA protein A affinity resin opens up a large window of operation for prepacked columns for mAb purification. Using the ReadyToProcess column format, the improved capacity of MabSelect PrismA enables purification of mAbs from bioreactor harvests of even higher titers than what was possible with its predecessor products.
MATCHING THE NEEDS OF UPSTREAM TITERS FOR OPTIMAL PROCESS ECONOMY
Column diameter, bed height, and resin characteristics determine total column capacity. Hence, for a given diameter, the bed height might need to be varied to optimally accommodate different upstream product titers.
Increasing the bed height from 20 to 25 cm at a given column diameter can increase the total column capacity by 25%.
Increasing the bed height makes it possible to process the same harvest in fewer cycles, ultimately reducing process time (Table 1). When scaling, however, the load residence time should be kept constant by adapting the flow velocity.
To address process economy in commercial manufacturing, reusing the prepacked columns for several manufacturing campaigns can be an attractive option. A study with real harvest feed shows that the disposable ReadyToProcess columns can be used for up to 50 cycles with maintained performance, making these columns a viable option for large-scale regular manufacturing.
Tabel 1. The amount of mAb that a ReadyToProcess column with 359 mm i.d. can process in a single cycle
(data for two different chromatography resins packed at three bed heights)
| Bed height (mm) | Load (g/L) | Processed mAb (g/cycle) | ||
| MabSelect SuRe LX | MabSelect PrismA | MabSelect SuRe LX | MabSelect PrismA | |
| 150 | 47 | 63 | 705 | 954 |
| 200 | 47 | 63 | 940 | 1260 |
| 250 | 47 | 63 | 1175 | 1575 |
SINGLE-USE CHROMATOGRAPHY SYSTEMS WITH INCREASED FLOW RATES
The benefits of single-use technologies at manufacturing scale are also attractive from a hardware perspective, as cleaning procedures can be simplified and start up time when setting up a new facility can be shortened. In the downstream space, there has been a limitation in the availability of single-use chromatography systems that can handle high flow rates. With ÄKTA ready XL, this has changed. The single-use ÄKTA ready XL chromatography system is designed for manufacturing scale and can meet the capacity demands from single-use upstream processes of 2000 L high-titer feeds. The possibility of using two flow kit sizes that cover a broad range of flow rates from 45 to 3500 L/h enables operation of large-scale columns with inner diameters of up to 1200 mm, bringing single-use chromatography to large manufacturing scale. By being part of a scalable single-use chromatography platform, ÄKTA ready XL simplifies technology transfer and scale-up (Fig 1).
Fig 1. ÄKTA ready and ÄKTA ready XL operate ReadyToProcess columns with inner diameters from 80 to 600 mm for purification of biomolecules from bioreactor culture volumes of 10–2000 L. For larger bioreactor volumes, ÄKTA ready XL can also operate AxiChrom columns with inner diameters of up to 1200 mm. The common UNICORN software platform simplifies transfer of processes between systems.
THE IMPORTANCE OF EASE OF USE
As the scale increases, so does the equipment size. Implementing single-use technologies will increase operator interaction. Hence, usability and design will be important, for example, to facilitate exchange of the flow kit. ÄKTA ready XL has a compact design and all system components are easily accessed for operator convenience. Flow kit installation is intuitively performed using the software-aided installation wizard. Markings on the system show where the tubing should be placed and sensors connected. Before start of the purification process, proper connection of the sensors is ensured by running an installation test. ÄKTA ready XL is movable and its small footprint allows for efficient facility use (Fig 2).
Fig 2. ÄKTA ready XL offers flexibility at manufacturing scale
MEETING THE RELIABILITY REQUIREMENTS FOR MANUFACTURING
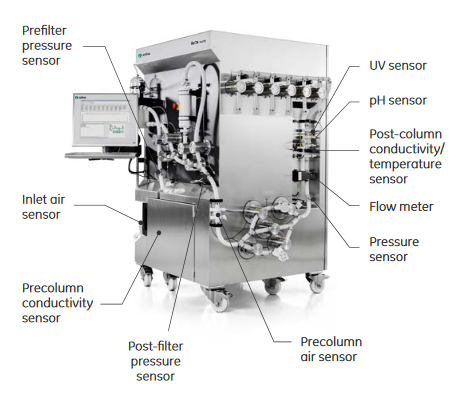
Monitoring of process performance is critical in biomanufacturing. Up until today, single-use sensor technology has been perceived as unreliable and limited to only a few analytes. These functional limitations are being constantly rewritten. With ÄKTA ready XL, all sensors included in the flow kits are based on single-use technology (Fig 3). The sensors show high accuracy for dependable monitoring and control of the purification process, and offer the reliability needed for GMP production (Table 2).
Together with precolumn air sensors at the inlet and in the flow path, the air trap automatically detects and prevents introduction of air into the column. Post-column sensors monitor the pressure, conductivity, temperature, pH, flow, and UV.
Fig 3. ÄKTA ready XL installed with flow kit featuring single-use sensors with high accuracy.
Table 2. Specifications of ÄKTA ready XL sensor technology
| Description | Range | Accuracy |
| Conductivity | 2–140 mS/cm | ± 0.15 mS/cm, or ± 7 of actual value* |
| Temperature | 10°C–30°C | ± 2°C |
| Pressure | 0.1–4 bar | ± 0.1 bar, or ± 5 % of actual value |
| Flow Kit ¾ inch i.d. | 45–1900 L/h | 45 to 300 L/h: 5% to 2%, 300 to 1900 L/h: 2% |
| Flow Kit, 1 inch i.d. | 90–3500 L/h | 90 to 600 L/h: 5% to 2% 600 to 3500 L/h: 2% |
| pH | 4-10 pH | ± 0.3 |
| UV | 0–1 AU | Linearity ± 2% |
*Accuracy can be improved to 3% using the calibration function of the UNICORN software.
SINGLE-USE TECHNOLOGIES AT MANUFACTURING SCALE
Single-use technologies are here to stay. While disposables are dominant in precommercial scale, employing single-use equipment is not as common in commercial manufacturing, especially not in the downstream space. This situation will likely change with more single-use technologies adapted to commercial production scale and when new single-use processes move through the development pipeline to commercial manufacturing.