When the biopharmaceutical industry began using disposable equipment, single-use films were made from off-the-shelf materials borrowed from other industries, such as food. However, a number of experiences over the last several years, such as the case with anti-oxidants as published in the PDA Journal of Pharmaceutical Science and Technology, have taught us that using materials not intended for use with pharmaceuticals was not the best choice. As a result, the industry is now evolving toward developing single-use films that are better suited to fit the needs of biomanufacturing. Specifically, end users have communicated a need for improved film performance with reliable supply, which requires transparency along the entire supply chain. Through open collaboration and communication with its customers, Cytiva was able to identify other common pain points and work with a reliable partner to develop a solution that adequately addresses those challenges.
Getting down to the basics
To develop an appropriate single-use film for the biopharmaceutical industry, it is important to look across all applications of single-use systems, from the upstream to the downstream unit operations, and determine the attributes a film must have that are critical to the quality of the products. For example, the environmental conditions (e.g., temperature, humidity, etc.), scale, duration, as well as static and dynamic forces at play with each application of single-use technology can be used to define the key performance requirements for the film. These attributes will then be used to guide resin selection and the architecture of the film. While the attributes may vary across applications due to single-use films serving multiple functions, the film must be, at the very least, composed of materials that are consistent, biocompatible, and chemically inert with low extractable levels. As a supplier, it is ideal to gain a complete understanding of the raw materials used for the film and the engineering and science behind them to develop an in depth understanding on how they contribute to film performance. With detailed information about a film and its components, the supplier can and should provide this information to the customer when requested.
As the biomanufacturing space matures and security of supply and transparency continue to remain at the forefront, end users should expect their suppliers to know specific details about their own partners and what quality control measures are in place for the film they are receiving from them. For example, they should know where the film is manufactured, what quality management system governs that location, and what capabilities are in place to characterize the chemical, physical, and mechanical properties of the material. Through open communication along the supply chain, end users can take comfort in knowing their own supplier is a trustworthy, reliable, and knowledgeable partner.
An optimal solution for bioprocessing needs
In an effort to create a film that meets customer needs and is also suitable for use across Cytiva's full single-use portfolio, Cytiva recently announced a new collaboration agreement with Sealed Air Corporation. As detailed in the release, both companies share a vision about the importance of using high-quality films in the efficient manufacturing of lifesaving medicines, such as monoclonal antibodies, vaccines, and next-generation biotherapeutics.
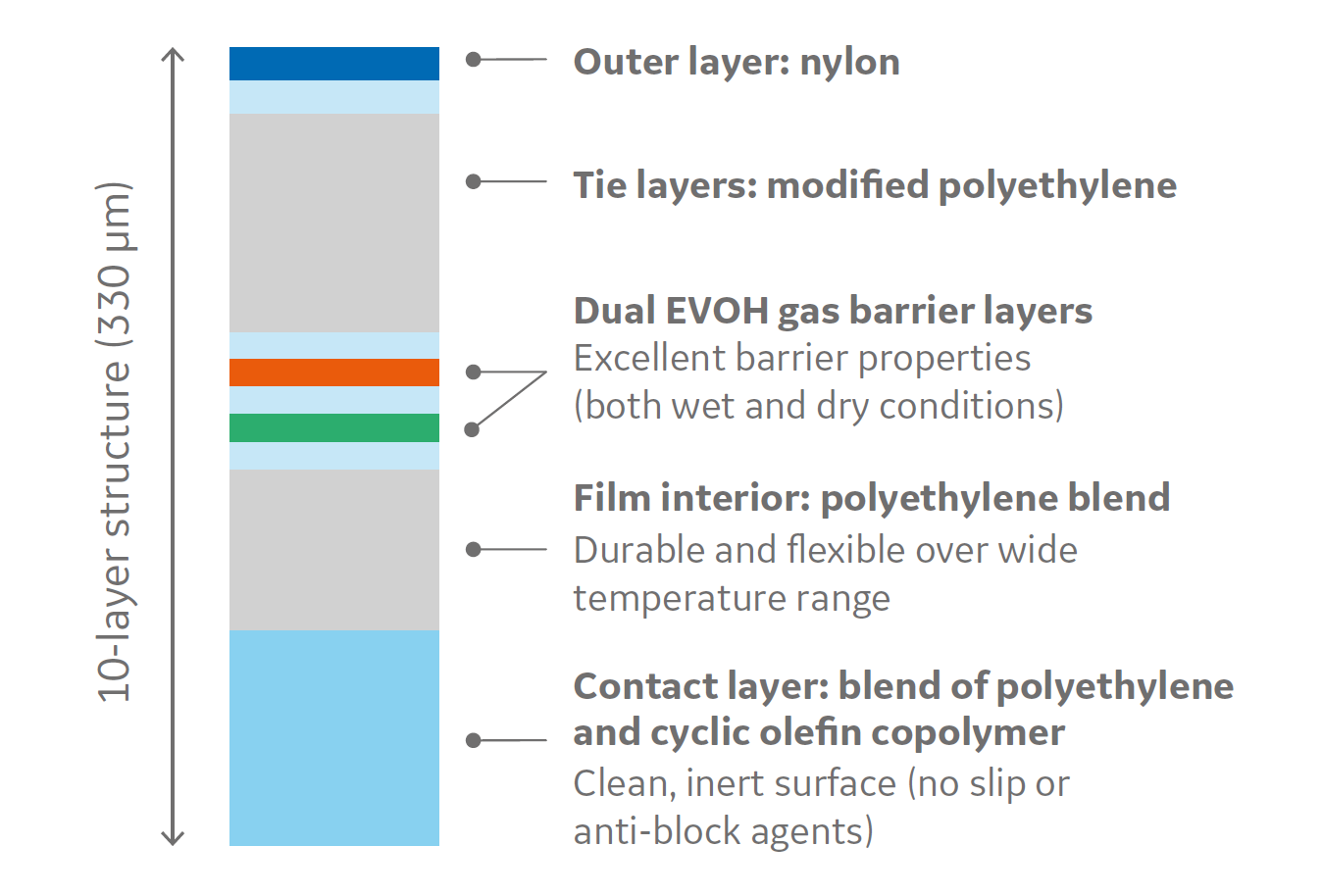
The design for the new film is a technology developed with Sealed Air that builds upon today’s existing design for single-use film. While most films have five to seven layers, Cytiva's new film includes a 10-layer structure detailed in the image below:
One reason Cytiva selected Sealed Air as its partner was their willingness to share extensive information about their materials, including information related to each of the layers in the film. This gives Cytiva the ability to explain the film’s unique qualities to its customers. For example, the contact layer is a blend of polyethylene and cyclic olefin copolymer. One key advantage is that it does not require small molecule slip agents, which can potentially become leachables. Cyclic olefin copolymers are currently used for pharmaceutical container technology in part due to their potential benefit of low protein adsorption.
In addition, the film includes two ethylene vinyl alcohol (EVOH) copolymer layers. The reason for using two types of EVOH is to help achieve good gas barrier properties, such as biologic/product stability, in both wet and dry conditions to support applications with different humidity controls. Nylon was selected as the outer layer because this resin offers good performance under the humid conditions the film would experience in bioprocess applications. Finally, there is a polyethylene blend interior that is durable and flexible over a wide temperature range, which the film will be exposed to in different applications across unit operations.
A major benefit to Cytiva's new film is that the less flexible resin layers are in the interior of the film, where forces associated with flexural fatigue are neutralized. By having them there, there is an ability to maintain gas barrier properties during more physically challenging applications such as the transport of bulk liquids. These are the scientific details a supplier must be able to understand, establish, and control so they can explain these benefits to customers.
Going the extra mile
Born of customer collaboration, supplier partnership, and Cytiva's bioprocessing expertise, the new film offers the simplicity of qualifying one film across the full range of single-use bioprocessing systems, saving time, effort, and cost. As it is tested and evaluated, Cytiva is paying close attention to all the critical film attributes and using test methodologies from recognized industry organizations, such as BPOG, ASTM, ISO, and USP, as it is important to be able to offer customers data around these guidelines. In addition, Cytiva's scientists and engineers have designed new test methodologies to gain a deeper understanding of attributes such as film flexural properties and abrasion resistance to ensure strong performance under demanding application conditions.
Cytiva has also implemented chemical identification and cell culture testing with each lot of film to provide an additional level of assurance of reproducibility and predictability of the film.
The film has been tested in the most rigorous applications to seek answers to questions like:
- Can it support large volumes while still maintaining integrity?
- Will it be able to remain integral when it is exposed to freezing conditions?
- Is it going to be able to endure high temperatures and long-term storage?
- Is it strong enough for bulk liquid transport?
- Can it resist flexural fatigue during the wave rocking motion in bioreactors?
All of these factors come into play when it comes to large-scale plastic containers, so it is important a film can withstand various strenuous conditions to ensure the physical and mechanical properties are well-suited for biopharmaceutical manufacturing. The overall goal is to design a film for bioprocess applications that can keep up with the latest industry needs and ultimately be the cornerstone for future developments.
Fortem film was born of customer collaboration, supplier partnership, and Cytiva's bioprocessing expertise. It offers the simplicity of qualifying one film across the full range of your single-use bioprocessing systems, saving you time, effort, and cost. Learn more...
- Watch the video: Fortem single-use bioprocess film – Cytiva introduces one film for its entire portfolio of single-use bioprocess systems
- Download the poster: Resin selection to optimize the flexural strength of bioprocess film
- View the brochure: The many strengths of one film – Fortem – a single-use platform film built for bioprocess
- Request a free sample: Evaluation kit includes three 2 L Cellbag bioreactor chambers made of Fortem film. Cellbags are commonly used in the WAVE 25 Bioreactor system. Each bag measures 559 mm (22.00 in.) x 267 mm (10.51 in.), is manufactured from a unique lot, and is gamma irradiated.