Affinity chromatography
What is affinity chromatography?
Affinity chromatography (AC) separates proteins on the basis of a reversible interaction between the target protein and a specific ligand attached to a chromatography base matrix.
The interaction can be biospecific, for example, antibodies binding protein A or a receptor binding a hormone; or nonbiospecific, for example, a protein binding a dye substance or histidine-containing proteins binding metal ions as in immobilized metal ion affinity chromatography (IMAC).
How does affinity chromatography work?
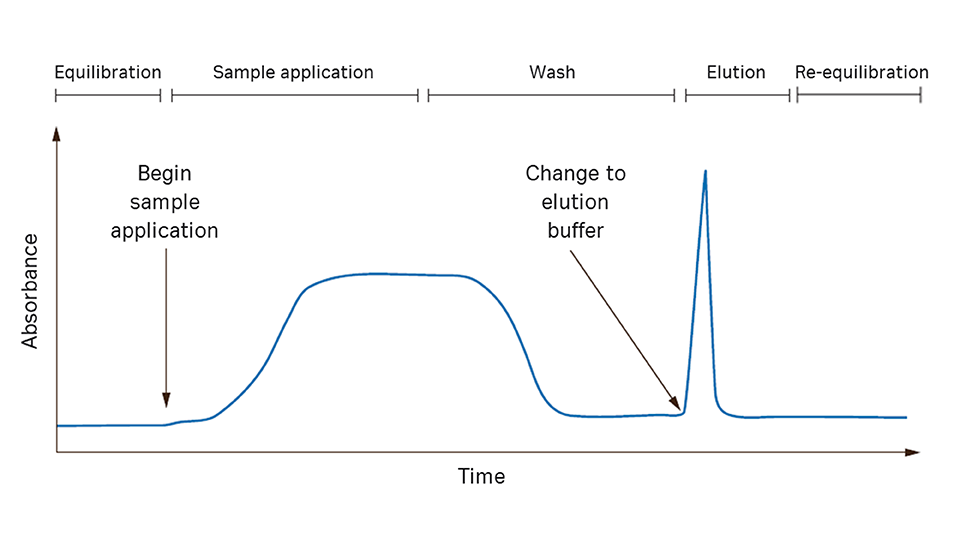
In affinity chromatography (the target protein is specifically and reversibly bound by a complementary binding substance (ligand). The sample is applied under conditions that favor specific binding to the ligand. Unbound material is washed out of the column.
The bound target protein is recovered by changing conditions to those favoring elution. Elution is performed specifically, using a competitive ligand, or nonspecifically, by changing, for example, pH, ionic strength, or polarity. The target protein is eluted in a purified and concentrated form.
When should I use affinity chromatography?
Because of its high selectivity, affinity chromatography can be used for single-step purifications. More commonly, however, AC is used as the first capture step, followed by one or more polishing steps to remove remaining impurities.
For example, protein A or protein G ligands coupled to an agarose base matrix are used for routine purification of antibodies.
Today, most laboratory-scale purifications are performed with affinity-tagged proteins. Histidine-tagged proteins are purified using IMAC, and glutathione S-transferase (GST)-tagged proteins are purified using chromatography resin with immobilized glutathione.
Affinity chromatography is also used to remove specific impurities.