Quantitation of proteins part 2
In this series on protein quantitation in Western blotting (immunoblotting), part 1 discusses densitometry and its influencing factors. Here, part 2 discusses the common chemiluminescent, fluorescent, and radiolabeling detection methods suitable for quantitative analysis.
Quantitation using chemiluminescent detection
Enhanced chemiluminescence (ECL) relies on a horseradish peroxidase (HRP)-conjugated secondary antibody reacting with an ECL reagent, which contains substrate and luminol.
This reaction releases measurable light—detectable by X-ray film or digital imagers—proportional to protein concentration across a broad linear dynamic range.
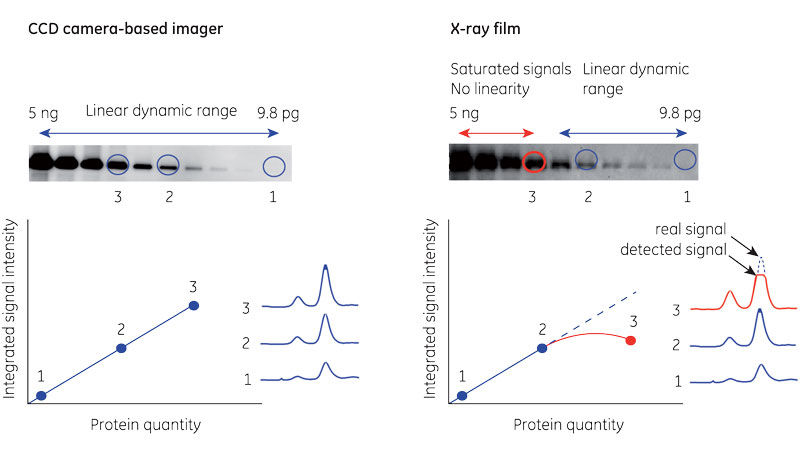
Generally, X-ray film is less suited to quantitation than digital imagers. Film acts as an intermediary between the membrane and quantitation, restricting linear dynamic range and making small differences in intensity appear larger. Film can also mask sample-to-sample variation when saturated by signal.
In contrast, digital Western blot imagers make chemiluminescence analysis straightforward. The imaging system captures signal, and software uses densitometry to quantitate. This method can provide an effective measure of the relative signal between protein bands across lanes.
Figure 1 demonstrates the difference in linear dynamic range between digital charge-coupled device (CCD) camera-based imaging and X-ray film.
Fig 1. Comparison between CCD camera and X-ray film in chemiluminescent band detection. All bands fall within the linear dynamic range for the CCD camera (A). The most intense bands cause saturation on the film (B).
Chemiluminescence also has limitations. True multiplexing is not possible, which means that normalizing signal to a reference protein is potentially inaccurate. Usually, the only options for targeting a reference protein are:
- Stripping and reprobing the membrane with another primary antibody against the reference protein.
- Running duplicate gels and transfers for target protein and reference separately.
- Using two primary antibodies raised in identical hosts for the target protein and reference.
These options can be challenging in practice, affecting reproducibility and adding time to protocols. The third option also requires sufficient resolution between target protein and the reference to image simultaneously.
Quantitation using fluorescent detection
Western blot detection via fluorescence uses fluorophore-conjugated secondary antibodies, avoiding the need for enzymatic reactions. A laser scanner or CCD camera-based system excites the fluorophores and collects the emitted light to produce an image.
Fluorescence detection is sensitive, stable, and reproducible. The method provides a broad dynamic range for accurate quantitation comparable among different experiments (Fig 2A).
Fluorescence also supports multiplexing, enabling imaging of multiple fluorophores simultaneously, including those of similar molecular weight (Fig 2B). Multiplexing simplifies normalization to an internal reference, removing the need for additional steps and potential loss of target protein through stripping.
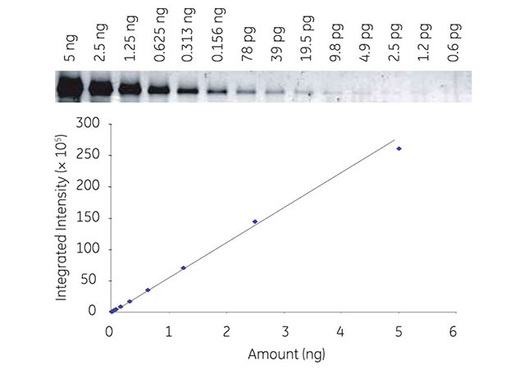
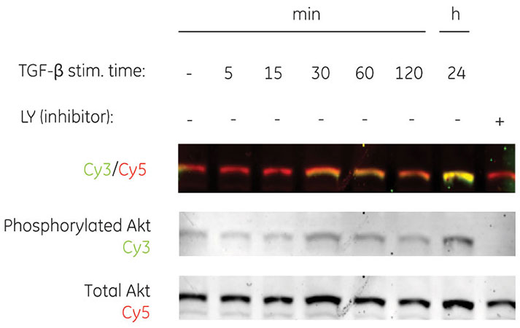
Fig 2. Series dilution of transferrin detected with Cy3 labeled secondary antibodies by fluorescence, demonstrating broad dynamic range with Amersham ECL Plex (A). Multiplex detection of two forms of human Akt protein (phosphorylated and total) using Amersham ECL Plex (B).
2-D Fluorescence Difference Gel Electrophoresis (2-D DIGE) is an example of a technique that uses fluorescence multiplexing for normalization. An internal standard for every spot on the gel minimizes gel-to-gel variation, allowing comparison and densitometry of samples across multiple gels.
Quantitation in radiolabeling applications
Although there are safety considerations for handling radiolabeled membranes, a radioisotope provides a sensitive and stable signal over its predictable half-life.
Traditionally, radiolabeled antibodies used for immunoblotting required X-ray film for detection. Although still in common use, this method suffers from the same drawbacks as chemiluminescence in terms of linear dynamic range.
Digital imaging systems, such as Typhoon biomolecular imager, offer phosphorimaging as one of multiple imaging modes. This mode provides an alternative method to detect radioactivity, using phosphor screens as an intermediary.
Unlike film, phosphor screens enable sensitive detection and quantitation of proteins across a broad linear dynamic range. Imaging systems interpret the photostimulated luminescence, converting it into quantitative data.
These imaging systems have analysis software built in, and can guide users through imaging and densitometric analysis.
How do I select the most appropriate detection method?
Experimental goals and quantitation requirements vary from one project to another. Users can benefit from comparing individual experiment or project needs to the benefits and limitations of each detection method.
The preferred method would provide sufficient sensitivity and linear dynamic range for the experiment. Across multiple experiments, signal stability enables comparisons among blots conducted at different times.
For all immunoblotting methods, the choice of antibody will contribute to the level of nonspecific background. However, digital imaging systems allow users to manage this signal-to-noise ratio, and simplify the process from imaging to analysis.
For more hints and tips, download the Imaging and and Western blotting principles and methods handbooks.