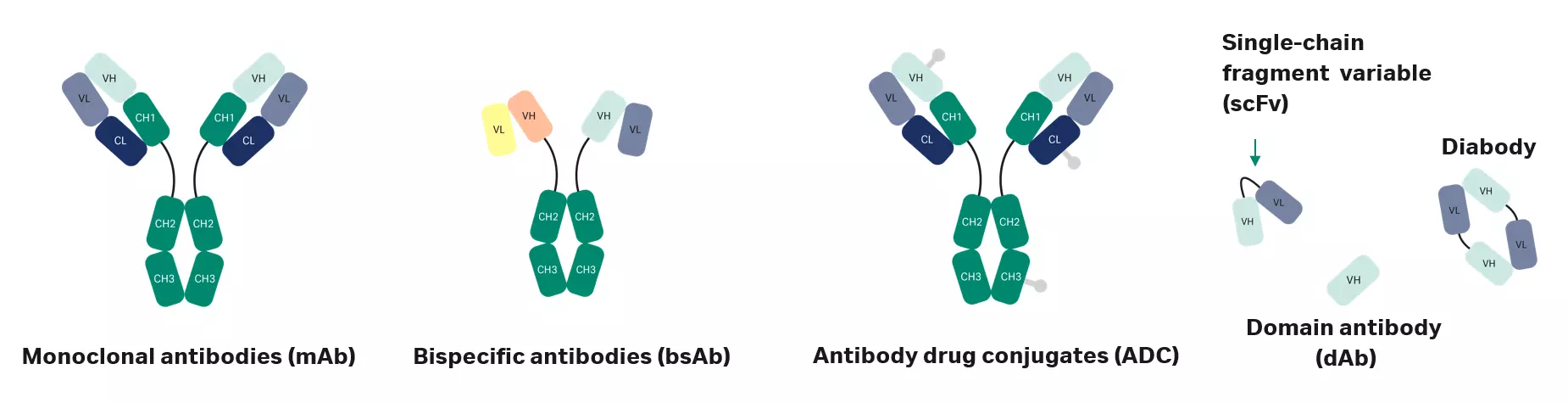
There is a vast array of antibody variants, including multispecific (msAb), bispecific antibodies (bsAb), antibody fragments (Fab, dAb, scFv), and antibody-drug conjugates (ADC). But with this diversity comes challenges.
Antibody therapeutics are the largest class of biotherapeutics, and their development has been ongoing for decades. The first antibody therapeutic drug, which treated transplant rejection, was approved in 1986. Over the years, development beyond traditional monoclonal antibodies (mAb) has increased. Scientific progress and protein engineering capabilities have unleashed a large variation in different types of antibody variants such as multispecific (msAb), bispecific antibodies (bsAb), antibody fragments (Fab, dAb, scFv), and antibody drug conjugates (ADC).
Developing purification processes for antibody variants is not always straightforward and the platform approaches conveniently used for many traditional mAbs cannot be used.
Developing purification protocols for the diversified therapeutic antibody pipelines
Production of monoclonal antibodies commonly includes platform processes to make the development and manufacturing standardized and process development quicker. With the diverse molecular pipeline, an existing platform may not work, and there is a need to develop new platforms. Depending on the structure of the target antibody and the impurity profile, other affinities, like protein L or variants of protein A, may improve purity and increase removal of impurities. The goal is to remove as many impurities as possible in the capture step before continuing to the polishings steps.
Common mAb process-related impurities must be addressed in the polishing steps along with product-related impurities that are present for antibody variants. Therefore, techniques like multimodal chromatography (MM) or hydrophobic interaction chromatography (HIC) may be needed instead of or in addition to conventional ion exchange chromatography (IEX). Multimodal (or mixed mode) resin interactions includes both ionic exchange and hydrophobic interactions and have been shown efficient for aggregate removal.
Safety and efficiency requirement as we know from traditional mabs
The demands for manufacturing are largely the same as for conventional mAbs. Impurities must be removed in the chromatography steps to specified levels, and the resins must withstand cleaning agents to avoid bioburden incidents. The target molecule needs to be stable over the process and in storage to reduce aggregation and degradation otherwise low yield and inefficient processes will result. Resins must be available and applied in research-scale formats to process development and manufacturing formats for predictable and smooth scale-up.
Cytiva helps with expertise and new innovations
Cytiva assesses how we can support the molecular pipeline by listening to process developers and manufacturers. We listen to their needs and challenges. Based on this real-world knowledge, we determine what elements to include in our product development to improve efficiency for our customers. When it comes to supporting the antibody variants pipeline, Cytiva recently announced the MabSelect™ VH3 affinity resin. This resin uses an engineered protein A ligand that interacts only with the variable heavy chain containing the VH3 sequence family. MabSelect™ VH3 offers high binding capacity for antibody fragments and provides excellent separation of bispecific antibodies containing VH3 sequence family. The resin is an important tool in the Cytiva resins toolbox and follows on the launch of MabSelect PrismA™ and MabSelect™ VL resins.
“We are proud to announce the MabSelect™ VH3 resins and excited to support our customers working with bispecific antibodies and antibody fragments. This resin is an excellent example of how the needs of our customers have determined our product development. A collaboration between our experienced R&D team and our users has resulted in development of a resin that brings efficiency and robustness to processing of antibody variants.”
Henrik Ihre, Business Leader – Next Generation Resins & Technologies, Cytiva
Learn more about the Cytiva high-flow, modern affinity resins in the toolbox including MabSelect™ VH3, MabSelect PrismA™, MabSelect VL resins, and Capto™ polishing resins.
We have an experienced team of scientists around the globe to support in the development of purification protocols for mAb and antibody variants. If you like to get in contact with them, let us know.