Introduction
Personalized medicine. Once just an idea, personalized medicine is becoming a reality. This realization is thanks in part to developments in molecular diagnostics. The rapid development and growth in the field has also been propelled by pioneering techniques and the effects of the COVID-19 pandemic.
Discover more about the impacts of COVID-19 on molecular diagnostics
Emerging technologies such as isothermal amplification, mass spectrometry methods, and methylation analysis enable faster and more personalized approaches to patient healthcare. With applications across infectious diseases, oncology, and genetics, advances in molecular diagnostics are paving the way for earlier detection of diseases, more targeted therapies, and improved patient outcomes.
Mass spectrometry: from biomarker detection to healthcare revolution
Mass spectrometry (MS) is a powerful analytical technology with high sensitivity and high specificity. While it has mainly been used for analytical chemistry in the past, its applications have expanded significantly in recent years, extending into molecular diagnostics and healthcare. MS can precisely identify and quantify biomolecules in a variety of biological specimens, providing high-quality quantitative analysis even at low concentrations (1).
MS has several advantages over traditional techniques due to its versatility, multiplexing capacity, and remarkable analytical specificity and sensitivity (2). However, it requires rigorous sample preparation or purification processes, in addition to being highly technical, and generally very costly. Current progress is focused on addressing these issues to produce MS systems that can provide fast, affordable, portable, and high-throughput analysis of low-volume samples (3).
MS techniques, particularly MS-based clinical proteomics, have diverse applications in diagnostics across various medical fields, being used in biomarker discovery for early detection and prognosis, and identifying markers for treatment response prediction and monitoring (1).
Recent advancements have led to the approval of several MS-based in vitro diagnostic methods by the US Food and Drug Administration (FDA) (45). These tests encompass pathogen identification, newborn screening, quantification of therapeutic drugs in the bloodstream, and a vitamin D assay, marking significant milestones in enhancing clinical diagnostics and patient care.
Isothermal amplification: going beyond the lab
Historically, nucleic acid amplification tests (NAATs) have been largely considered highly complex and costly and, as a result, have been limited to molecular laboratories staffed with skilled technologists. With the emergence of COVID-19, molecular diagnostics have undergone many improvements, with the increased need for rapid and precise testing that can be done at the point-of-care (POC).
Isothermal amplification (IA) techniques amplify nucleic acids at a constant temperature and do not require the use of thermocycling equipment. IA methods, such as loop-mediated isothermal amplification (LAMP) and recombinase polymerase amplification (RPA), rely on enzymatic reactions to exponentially amplify target nucleic acid sequences. These techniques are ideal for POC diagnostics because they can make kits rapid and easy to use (5).
Read our previous blog to learn about qPCR
IA systems have widespread utility across a diverse array of applications, ranging from cancer diagnostics to the diagnosis of various infectious diseases, as well as the detection of single-nucleotide polymorphisms (SNPs) (6–12). Among these techniques, LAMP is the most established, with outstanding specificity and rapid turnaround times (13). This method has also been approved by the World Health Organization (WHO) as an alternative molecular diagnostics method for pulmonary tuberculosis and SARS-CoV-2 (14–15).
Despite this, IA approaches do face some challenges such as complex primer design, nonspecific amplification, and difficulty amplifying low concentrations. However, the integration with other technologies like nanotechnology and microfluidics will significantly enhance their sensitivity, specificity, and scalability, opening new frontiers in disease diagnostics and monitoring.
DNA methylation analysis – taking personalization one step further
DNA methylation is a key epigenetic modification involved in the regulation of gene expression and chromatin structure, playing a critical role in diverse biological processes, including development, differentiation, and disease (16). In molecular diagnostics, DNA methylation analysis has emerged as a powerful tool for understanding disease pathogenesis, predicting patient outcomes, and guiding personalized therapeutic strategies (17).
Comprehensive genome-wide methylation assays, facilitated by microarrays and next-generation sequencing (NGS), can provide novel insights into the functional impact of methylation profiles. Tissue-specific and differentially methylated regions are found throughout the genome, with changes in methylation patterns implicated in a diverse array of physiological and pathological processes.
Discover more about microarrays and NGS
DNA methylation analysis is widely used in cancer research for tumor classification, risk stratification, and treatment selection. Methylation-based biomarkers have been shown to distinguish between different cancer types and subtypes, predict patient survival, and determine individual response to therapy (18–22). Furthermore, DNA methylation profiling is integral to the development of liquid biopsy assays for the noninvasive detection of circulating tumor DNA (ctDNA) in blood or other bodily fluids, enabling early cancer diagnosis and real-time monitoring of treatment response and disease progression (23).
In the future, DNA methylation signatures could be used to tailor treatment strategies based on individual patient characteristics, optimizing therapeutic efficacy, and minimizing adverse effects. However, this ability relies on the further standardization and validation of DNA methylation assays for clinical use, along with the development of user-friendly bioinformatics tools for data analysis and interpretation.
Don’t forget sample preparation
Without question, the future of molecular testing is rapidly evolving to become more personalized and portable, providing rapid and comprehensive results where they are needed. As the field of molecular diagnostics continues to improve, it’s essential that sample preparation techniques evolve in tandem. Many of the biggest challenges in using molecular diagnostic tests today are related to sample preparation, including low concentration of analytes, the presence of interfering substances, and complex and time-consuming protocols.
Magnetic beads, such as Sera-Mag™ beads and speedbeads, provide a simple and reliable method of purifying various types of biomolecules directly from samples, including genomic DNA, plasmids, mitochondrial DNA, RNA, and proteins (Fig 1). While traditional methods often require time-consuming steps and expensive equipment, magnetic separation avoids these problems and is simple to use. With a cauliflower-shaped surface and proprietary chemistry, Sera-Mag™ particles demonstrate high binding capacity, consistent results, and adaptability, making them ideal for a range of diagnostic applications.
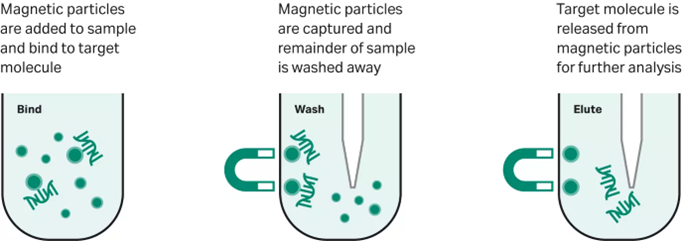
Fig 1. Overview of a magnetic bead-based workflow using Sera-Mag™ beads.
Coming to a future near you
The escalating impacts of an ageing population, disease pandemics, and antibiotic resistance have positioned molecular diagnostics at the forefront of modern healthcare. The rapid growth of molecular diagnostics has catalyzed the development of high-throughput assays that prioritize portability, speed, and personalization, ultimately aiming to deliver precise and timely results that significantly improve patient outcomes.
With mass spectrometry, isothermal techniques, and methylation analysis, the future of molecular diagnostics is looking brighter than ever. These cutting-edge technologies empower healthcare professionals with precise tools for early disease detection, personalized treatment strategies, and the real-time monitoring of patient responses. By harnessing the power of molecular insights, we are poised to address the complex healthcare challenges of today and tomorrow, ensuring better outcomes and quality of life for all.
For more in-depth detail on existing and emerging molecular diagnostics, read our white paper
- Birhanu AG. Mass spectrometry-based proteomics as an emerging tool in clinical laboratories. Clinical Proteomics. 2023;20(1):32. doi:10.1186/s12014-023-09424-x
- Fung AWS, Sugumar V, Ren AH, Kulasingam V. Emerging role of clinical mass spectrometry in pathology. Journal of Clinical Pathology. 2020;73(2):61-69. doi: 10.1136/jclinpath-2019-206269
- Zhou X, Zhang W, Ouyang Z. Recent advances in on-site mass spectrometry analysis for clinical applications. Trends in Analytical Chemistry. 2022;149:116548. doi: 10.1016/j.trac.2022.116548
- Banerjee S. Empowering Clinical Diagnostics with Mass Spectrometry. ACS Omega. 2020;5(5):2041-2048. doi: 10.1021/acsomega.9b03764
- Zanoli LM, Spoto G. Isothermal Amplification Methods for the Detection of Nucleic Acids in Microfluidic Devices. Biosensors (Basel). 2012;3(1):18-43. doi: 10.3390/bios3010018
- Horibe D, Ochiai T, Shimada H, et al. Rapid detection of metastasis of gastric cancer using reverse transcription loop-mediated isothermal amplification. International Journal of Cancer. 2007;120(5):1063-1069. doi: 10.1002/ijc.22397
- Srividya A, Maiti B, Chakraborty A, Chakraborty G. Loop Mediated Isothermal Amplification: A Promising Tool for Screening Genetic Mutations. Molecular Diagnosis & Therapy. 2019;23(6):723-733. doi: 10.1007/s40291-019-00422-0
- Schopf E, Liu Y, Deng JC, Yang S, Cheng G, Chen Y. Tuberculosis detection via rolling circle amplification. Analytical Methods. 2011;3(2):267-273. doi: 10.1039/c0ay00529k
- Rohrman BA, Richards-Kortum RR. A paper and plastic device for performing recombinase polymerase amplification of HIV DNA. Lab on a Chip. 2012;12(17):3082-3088. doi: 10.1039/c2lc40423k
- Chow WHA, McCloskey C, Tong Y, et al. Application of Isothermal Helicase-Dependent Amplification with a Disposable Detection Device in a Simple Sensitive Stool Test for Toxigenic Clostridium difficile. The Journal of Molecular Diagnostics. 2008;10(5):452-458. doi: 10.2353/jmoldx.2008.080008
- Krõlov K, Frolova J, Tudoran O, et al. Sensitive and Rapid Detection of Chlamydia trachomatis by Recombinase Polymerase Amplification Directly from Urine Samples. The Journal of Molecular Diagnostics. 2014;16(1):127-135. doi: 10.1016/j.jmoldx.2013.08.003
- Li J, Deng T, Chu X, et al. Rolling circle amplification combined with gold nanoparticle aggregates for highly sensitive identification of single-nucleotide polymorphisms. Analytical Chemistry. 2010;82(7):2811-2816. doi: 10.1021/ac100336n
- Oliveira BB, Veigas B, Baptista PV. Isothermal Amplification of Nucleic Acids: The Race for the Next “Gold Standard.” Frontiers in Sensors. 2021;2. doi:10.3389/fsens.2021.752600
- The use of loop-mediated isothermal amplification (TB-LAMP) for the diagnosis of pulmonary tuberculosis: policy guidance. Accessed February 26, 2024. https://www.who.int/publications-detail-redirect/9789241511186
- Kashir J, Yaqinuddin A. Loop mediated isothermal amplification (LAMP) assays as a rapid diagnostic for COVID-19. Medical Hypotheses. 2020;141:109786. doi: 10.1016/j.mehy.2020.109786
- Kiselev IS, Kulakova OG, Boyko AN, Favorova OO. DNA Methylation As an Epigenetic Mechanism in the Development of Multiple Sclerosis. Acta Naturae. 2021;13(2):45. doi: 10.32607/actanaturae.11043
- Galbraith K, Snuderl M. DNA methylation as a diagnostic tool. Acta Neuropathologica Communications. 2022;10(1):71. doi: 10.1186/s40478-022-01371-2
- Draškovič T, Hauptman N. Discovery of novel DNA methylation biomarker panels for the diagnosis and differentiation between common adenocarcinomas and their liver metastases. Scientific Reports. 2024;14(1):3095. doi: 10.1038/s41598-024-53754-1
- Chen W, Zhuang J, Wang PP, et al. DNA methylation-based classification and identification of renal cell carcinoma prognosis-subgroups. Cancer Cell International. 2019;19(1):185. doi: 10.1186/s12935-019-0900-4
- Wu ZH, Tang Y, Zhou Y. DNA Methylation Based Molecular Subtypes Predict Prognosis in Breast Cancer Patients. Cancer Control. 2021;28:1073274820988519. doi: 10.1177/1073274820988519
- Lu CY, Hsiao CY, Peng PJ, et al. DNA Methylation Biomarkers as Prediction Tools for Therapeutic Response and Prognosis in Intermediate-Stage Hepatocellular Carcinoma. Cancers (Basel). 2023;15(18):4465. doi: 10.3390/cancers15184465
- Filipski K, Scherer M, Zeiner KN, et al. DNA methylation-based prediction of response to immune checkpoint inhibition in metastatic melanoma. Journal for Immunotherapy of Cancer. 2021;9(7):e002226. doi: 10.1136/jitc-2020-002226
- Gai W, Sun K. Epigenetic Biomarkers in Cell-Free DNA and Applications in Liquid Biopsy. Genes. 2019;10(1):32. doi: 10.3390/genes10010032