Molecular diagnostics is the branch of diagnostics that uses the techniques of molecular biology to diagnose disease, predict disease course, select treatments, and monitor the effectiveness of therapies. Molecular diagnostics uses molecular biology techniques to amplify and detect DNA and RNA sequences of human, viral, or microbial genomes. The general public became more aware of molecular diagnostics during the COVID-19 pandemic as they got PCR tests for the disease. But molecular diagnostics has been around for much longer. Genetic testing for inherited diseases such as sickle cell disease and screening for ovarian cancer mutations in BRAC1 and BRAC2 genes are other common examples of molecular diagnostics.
Molecular diagnostics has been a game-changer in healthcare, transforming the way we detect, diagnose, and monitor diseases. As technology advances, the field continues to evolve, with new and innovative approaches taking center stage.
Next-generation sequencing accelerates molecular diagnostics
After the completion of the Human Genome Project, a new wave of technologies, called next-generation sequencing (NGS), came onto the market. These sequencers use massively parallel sequencing of short reads for high throughput. NGS enables whole-genome, whole-exome, transcriptome, and targeted sequencing with relative ease. This technology allows for the rapid and cost-effective sequencing of entire genomes, enabling a deeper understanding of genetic variations, identifying disease-causing mutations, and paving the way for personalized medicine.
Molecular diagnostics benefits from the high throughput, speed, and resolution of NGS. One of the biggest areas for NGS in diagnostics is in noninvasive prenatal testing where NGS techniques are used. NGS techniques are used to analyze cell-free fetal DNA found circulating in the mother’s blood. But the use of NGS continues to grow, particularly in oncology, Mendelian diseases, complex diseases, and infectious diseases. The speed at which NGS will grow in different clinical markets will probably depend on pushing sequencing costs down further, as well as developments in the regulatory landscape. As clinical scientists get more familiar with the possibilities with NGS, they can also contribute to higher growth through demand.
Liquid biopsies for noninvasive diagnoses
Of course, to analyze a patient’s DNA or RNA, you need to get a sample of it. Previously, doctors performed a surgical procedure to take a solid biopsy from a patient’s tissue by cutting into it or using a needle to extract cells. Today, some cancers can be diagnosed and monitored using liquid biopsies.
Liquid biopsy is a medical technique that has emerged as a noninvasive method for diagnosing and monitoring various diseases by analyzing biomarkers present in bodily fluids like blood, saliva, urine, and other bodily fluids. These tests analyze trace amounts of DNA, RNA, and proteins circulating in bodily fluids.
Liquid biopsies offer the potential for early detection of diseases, including cancer, by identifying specific biomarkers that indicate the presence of abnormal cells or genetic mutations. Because of their ease of collection, doctors can take samples many times during treatment. In this way, these biopsy tests enable ongoing monitoring of disease progression (1) and treatment effectiveness, providing a dynamic view of a patient's health status.
As more research is done, the use of liquid biopsies for molecular diagnostics is likely to grow.
Single-cell sequencing of pathogens for diagnoses
Single-cell sequencing has the power to characterize the genome and transcriptome of individual cells. Conventional sequencing approaches use many cells and lack the sensitivity to analyze individual or a small number of cells. In contrast, single-cell sequencing measures individual cellular heterogeneity and can distinguish a small sub-population of cells within a larger overall population.
Recently, researchers have been using single-cell sequencing to diagnose bacterial infections. Diagnosing bacterial infections traditionally involves culture-based procedures where bacteria are grown until they reach detectable levels; a process that can take days. Single-cell sequencing can cut diagnosis time down to hours by isolating a bacterium and then amplifying its DNA for detection. Single-cell sequencing also has the added benefit of being able to identify rare bacteria such as antibiotic-resistant bacteria (2).
Many single-cell sequencing techniques exist and you can download a handbook to optimize your workflow. You can also take an eLearning course on single-cell sequencing to learn more.
CRISPR-based diagnostics and the hope of point-of-care and at-home testing
Where testing happens is a hot topic in diagnostics with the goal of moving the testing closer to the patients whether it is done at point of care (POC), at home, or in remote locations. Moving testing closer to patients can lead to earlier detection of diseases and may help curb the spread of infectious diseases. Diagnostic kit developers often try to meet the ASSURED (or REASSURED) criteria set by the World Health Organization (WHO). ASSURED stands for affordable, sensitive, specific, user-friendly, rapid, equipment-free, and delivered, and REASSURED adds real-time connectivity and ease of specimen collection (3). But moving molecular diagnostics into the field can be difficult because doctors’ offices and people don’t have PCR machines lying around.
Researchers think that clustered regularly interspaced short palindromic repeats (CRISPR) systems could be the key to developing POC molecular diagnostic assays (4, 5). CRISPR systems are part of the microbial adaptive immune system that recognizes foreign nucleic acids. For diagnostics, CRISPR systems can be engineered to recognize any DNA or RNA molecule. So far, the focus of CRISPR diagnostics has been for the detection of infectious diseases including Zika, Ebola, and corona viruses, but it could also be used for detecting noninfectious diseases and identifying single-nucleotide polymorphisms (SNPs) and deletions in DNA (4).
There are hurdles to overcome before CRISPR technology can be widely used in molecular diagnostics including sample preparation and storage and handling. But overall, developments in this field look very promising.
Lab-on-a-chip for diagnostics
Lab-on-a-chip (LOC) is the overarching name given to tiny devices that integrate and automate multiple lab techniques. LOCs have been around for decades and have been valuable tools in bioengineering. But more recently, LOC devices have been developed for diagnostics(6). LOC technology often uses microfluidics but may also use nano- or millifluidics. Thus, this technology is ideal for POC diagnostics because of their low fluid needs for both samples and reagents. The devices also have fast analysis times because of better fluid transport and high surface area-to-volume ratios.
An example of an exciting use of LOC is portable device to detect sickle-cell disease in newborns. The device runs a tiny version of the electrophoresis test that is normally used for diagnosis. The platform, called Gazelle, is small, battery-operated, and can be used to diagnose other diseases like malaria (7).
In 2023, researchers from Iowa State University and Texas A&M University developed a LOC diagnostic sensor that they say is 10 times more sensitive than current methods. The sensor builds on loop-mediated isothermal amplification (LAMP), which is a technique that amplifies the DNA of pathogens. LAMP usually requires the DNA to be labeled with fluorescent dyes for detection. The newly developed chip can sense label-free LAMP products. So far, this device has only been used to detect potato blight microorganisms, but the researchers hope their technology will be used to detect other pathogens that cause diseases in humans, animals, and plants (8).
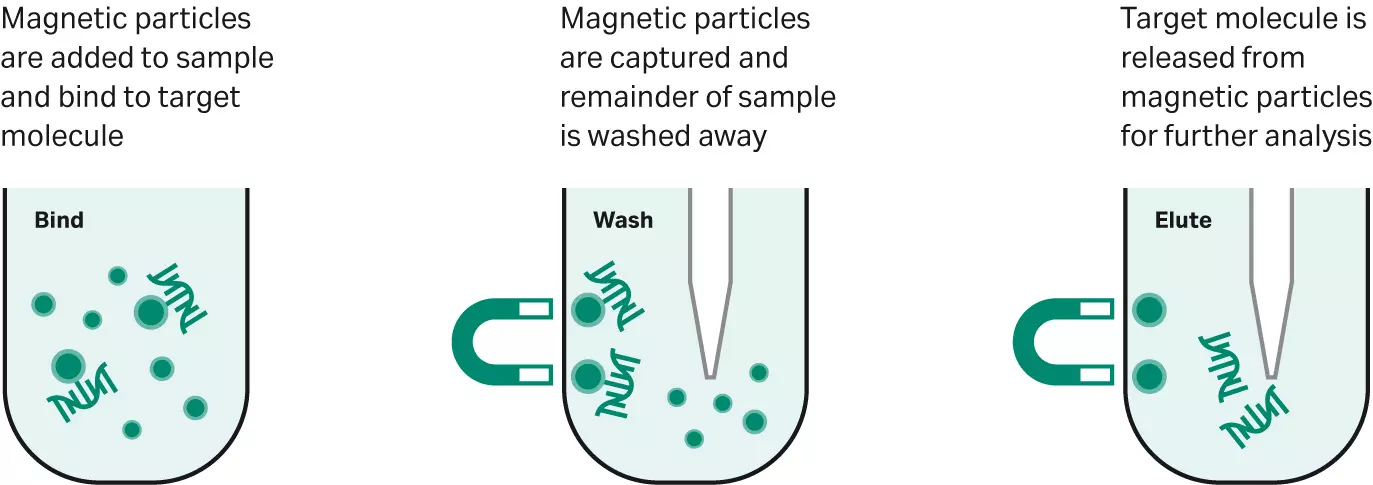
Magnetic beads for in-lab and POC diagnostics
Magnetic beads (magbeads) are not new in life sciences or in diagnostics. Magbeads can be used for the isolation and purification of cells, nucleic acids, proteins or any other biomolecules. The surface of magnetic beads can be coated with a variety of ligands to bind a specific target molecule. By exploiting this type of surface modification, magnetic beads offer an exceptionally versatile tool for biomolecule isolation.
Fig 1. A general magnetic bead workflow
For molecular diagnostics, magbeads are used primarily for sample preparation and molecular detection systems (9). Traditional methods of sample preparation such as centrifugation and filtration are time consuming and can damage biological samples. Magnetic separation avoids these problems and has the added benefit of ease of use. It does not require expensive equipment or specially trained people.
Using biosensors to detect biomolecules labeled with magnetic particles has already been used for in-lab diagnosis of Alzheimer’s disease, tuberculosis, sepsis, and other diseases. But there is also research on using magnetic beads in microfluidic, POC assay devices (9).
The versatility, specificity, and simplicity of magbeads makes them well-suited for molecular diagnostics. Beads are available with different chemistries, internal compositions, and sizes and can be customized to meet your criteria. Cytiva provides a broad range of magnetic beads for molecular applications, including a series of surface chemistries well-suited for magnetic immunoassay development. For support with any aspect of your assay development workflow, contact our Scientific Support team.
Conclusion
Molecular diagnostics is advancing at an astonishing pace, fueled by innovative technologies and methodologies. With NGS, single-cell sequencing, and CRISPR-based assays, molecular diagnostics is empowering healthcare professionals to make more accurate diagnoses and treatment decisions than ever before. Liquid biopsies are making sample collection easier, and CRISPR and LOC technologies and magnetic beads are poised to accelerate the development of POC and at-home assays. All these developments are opening new doors to early disease detection, tailored therapies, and improved patient outcomes, promising a brighter future for healthcare.
References
- Dawson SJ, Tsui DW, Murtaza M, et al. Analysis of circulating tumor DNA to monitor metastatic breast cancer. N Engl J Med. 2013;368(13):1199-1209. Doi:10.1056/NEJMoa1213261
- Li H, Hsieh K, Wong PK, Mach KE, Liao JC, Wang T-H. Single-Cell Pathogen Diagnostics for combating antibiotic resistance. Nat Rev Methods Primers. 2023;3(1). doi:10.1038/s43586-022-00190-y
- Land KJ, Boeras DI, Chen X-S, Ramsay AR, Peeling RW. Reassured diagnostics to inform disease control strategies, strengthen health systems and improve patient outcomes. Nat Microbiol. 2018;4(1):46-54. Doi:10.1038/s41564-018-0295-3
- Kaminski MM, Abudayyeh OO, Gootenberg JS, Zhang F, Collins JJ. CRISPR-based diagnostics. Nat Biomed Eng. 2021;5(7):643-656. Doi:10.1038/s41551-021-00760-7
- Ghouneimy A, Mahas A, Marsic T, Aman R, Mahfouz M. CRISPR-Based Diagnostics: Challenges and Potential Solutions toward Point-of-Care Applications. ACS Synth Biol. 2023;12(1):1-16. Doi:10.1021/acssynbio.2c00496
- Wu J, Dong M, Rigatto C, Liu Y, Lin F. Lab-on-chip technology for chronic disease diagnosis. npj Digital Med. 2018;1(1). doi:10.1038/s41746-017-0014-0
- Future of Medicine: Lab-on-a-chip devices starting to make an impact. National Heart Lung and Blood Institute. Accessed December 14, 2023. https://www.nhlbi.nih.gov/news/2021/future-medicine-lab-chip-devices-starting-make-impact.
- Mao S, Zhao J, Ding X, Vuong VA, Song J, Que L. Integrated Sensing Chip for ultrasensitive label-free detection of the products of loop-mediated isothermal amplification. ACS Sensors. 2023;8(6):2255-2262. doi:10.1021/acssensors.3c00227
- Chircov C, Grumezescu AM, Holban AM. Magnetic Particles for Advanced Molecular Diagnosis. Materials (Basel). 2019;12(13):2158. Published 2019 Jul 5. Doi:10.3390/ma12132158
Related content