Cell-based influenza virus production process using single-use equipment
The aim of this article is to demonstrate how Cytiva single-use products can be applied in the field of vaccine manufacturing. Including a brief discussion around modern vaccine processes, followed by a case study showing the scale-up of upstream and downstream processes for the production of a cell based live attenuated influenza virus using single-use ReadyToProcess technology (Fig 1).

Introduction
Egg-based production is the most commonly used technique for manufacturing of influenza vaccine. This technique has its limitation in that it requires one fertilized egg per produced vaccine dose. The objective of WHO’s global action plan (GAP) for influenza vaccines is to produce enough seasonal flu vaccine to immunize two billion people by 2015 (1). If facing a pandemic, the world would need more than 13 billion doses to protect a naïve population with two immunizations. To put that in perspective, in 2011, 25 of the world’s leading vaccine manufacturers produced 620 million doses (2). There is a clear need to produce more doses in a shorter time frame.
Cell-based vaccine production with well-characterized processes can be a solution to meeting the capacity requirements. It also has its advantage in shorter production time compared to egg-based production, allowing for an improved ability to more rapidly meet the seasonal strains.
There are three different types of flu vaccines on the market: split virus, subunit vaccines, and live attenuated. Live, attenuated influenza vaccine (LAIV) requires less virus particles per vaccine dose. This translates into more doses per production volume and can thus, to some extent, compensate for the lack in production capacity.
Several of the vaccines we consider to be the base in today’s Western immunization programs have not yet reached the developing world as there are many barriers to overcome, such as production cost, logistics, and production capacity. Diseases, such as Haemophilus influenza B (HiB), Japanese encephalitis (JE), rotavirus, and pneumococcal infections, are targets for the developing world to save millions of children’s lives. Hence, there is a continuing need to develop existing vaccine manufacturing processes to improve access for developing markets, for faster responses to the vaccine needs, and to increase the number of doses produced. Tomorrow’s vaccines need to be developed with new sets of technologies. By using modern techniques and cell-based upstream processes it is possible to produce closer to the local market to overcome distribution hurdles, as well as to bring down production costs and facility footprint.
Improved manufacturing agility and productivity
Biomanufacturing based on single-use technology offers great flexibility compared with traditional stainless steel facilities. Production lines can more easily be modified for the manufacture of different products and the adaptation of production volumes to meet market needs is significantly facilitated. Furthermore, disposables minimize the risk for cross-contamination between production batches, and the need for time-consuming and costly cleaning and validation procedures is made redundant.
Single-use systems are well accepted in today’s manufacturing industry and their widespread use is driven by benefits such as reduced and delayed capital investments as well as by a shift from fixed costs to variable costs. Single-use systems allow for quick changeover between campaigns with the opportunity to produce more batches per year. Optimized facility utilization is the key to minimizing production costs and potentially increasing revenue.
Reduced environmental impact
The environmental impact of implementing single-use products in the biomanufacturing process has been widely discussed. One perception is that the environmental impact is greater for disposables than for traditional stainless steel facilities. In a peer-reviewed life cycle assessment (LCA) study, performed by Cytiva in collaboration with Biopharm Services, single-use equipment was compared with conventional stainless steel equipment (3). The environmental impact across the entire life cycle of the product was assessed: from the production of the equipment’s constituent materials and components to the use and ultimate disposal of the equipment. The results from this study showed that a facility based on single-use equipment has lower environmental impact than a facility based on traditional stainless steel equipment. This was shown for all the 18 environmental impact categories investigated. The largest savings identified in the LCA study were shown to be energy demand and water requirements during the use phase.
Production of Influenza virus using single-use equipment
To meet the challenges in today’s vaccine manufacturing, a case study was conducted of a scaled-up vaccine production based solely on single-use equipment. Whole live influenza virus was used as model system. A summary of the production scale-up from laboratory scale is presented in this article. More detailed descriptions of the individual upstream and downstream processes are presented in the separate application notes 29043548 (4) and 29043549 (5).
Figure 2 gives an overview of the upstream and downstream processes including cell expansion, influenza propagation, purification, and analysis.

UPSTREAM PROCESSING
Adherent Vero cells were expanded in static cell factories to generate inoculum for 10 L seed cultures. The cells in the 10 L cultures were grown on Cytodex 1 microcarriers (3 g/L) in the WAVE Bioreactor 20/50 system. The cells were grown to a cell concentration of approximately 3 × 106 cells/mL. The cells were subsequently detached from the microcarriers with trypsin and used to seed a 50 L microcarrier culture in the WAVE Bioreactor 200 system. The microcarrier concentration was kept constant. Sufficient cell recoveries were obtained for a split ratio of 1:5 during scale up from 10 L to 50 L working volume.
A common challenge upon cell transfer is the lag phase, during which the cells are adjusting to the new culture medium and little or no increase in cell number is observed. In the 50 L cultivations, the cells reattached and started to grow on the new microcarriers without lag phase. The cell growth rate was shown to be similar in both the 10 L and 50 L cultures in three consecutive batches (Fig 3).
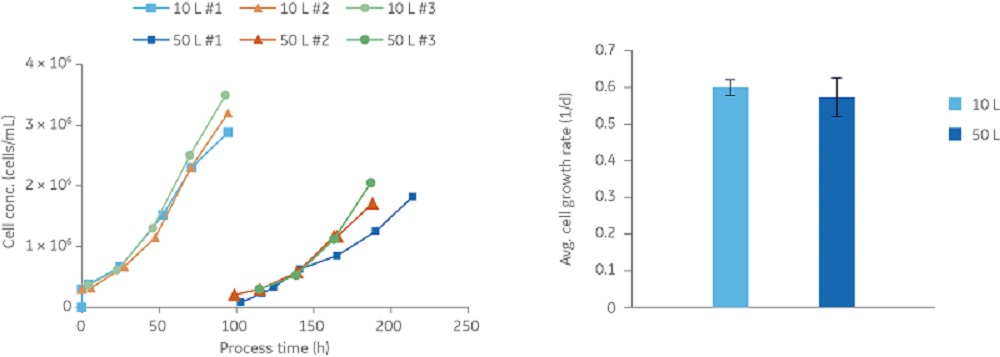
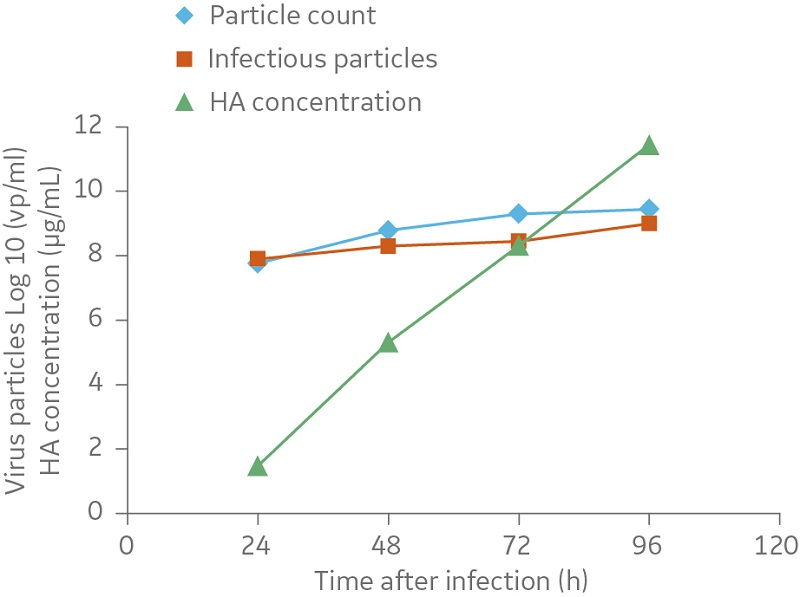
In the 50 L cultures, cells were grown to a cell density of approximately 2 × 106 cells/mL. The cells were infected with influenza virus strain A/H1N1/Solomon Island during the exponential growth phase at a multiplicity of infection of 4 × 10-3 and a trypsin concentration of 2 mg/L. Samples were taken daily during the infection phase. As shown in Figure 4, the infectious and total amount of virus particles initially increased quickly and then remained at a constant level until time of harvest, when the amount of virus particles was determined to be approximately 109 virus particles/mL.
The haemagglutinin (HA) concentration increased steadily throughout the infection phase and was approximately 12 μg/mL at time of harvest. Similar virus titers and HA concentrations were measured in the 10 L culture (data not shown) as in the 50 L culture (Fig 4).
A more detailed description of the cell culturing and virus infection and harvest is given in the application note 29043548 (4).
DOWNSTREAM PROCESSING AND PURITY ANALYSIS
For downstream laboratory-scale purification, performed using standard, non-disposable equipment, approximately 10 L virus harvest from the upstream processing was used. Approximately 44 L virus harvest from the 50 L (remaining 6 L corresponded to microcarrier volume) culture was used for further downstream processing with ReadyToProcess equipment in the scaled-up purification process.
Impurities originating from the host cells were removed during the downstream process. Cell debris was removed in the first microfiltration step using ULTA Prime GF 2 μm and 0.6 μm normal flow filters, followed by host cell DNA removal using a ReadyToProcess Capto Q chromatography column. Host cell proteins were removed using a ReadyToProcess Capto Core 700 chromatography column.
Capto Core 700 chromatography resin was used in the second chromatography step of the purification process (6). This chromatography resin has an inert outer layer of cross-linked agarose preventing molecules with a molecular weight (Mr) larger than approximately Mr 700 000 to enter the core of the bead. The bead core contains octylamine ligands that bind a broad range of substances including proteins, peptides, and nucleotide fragments. The multimodal function of the chromatography resin, utilizing both size exclusion and affinity binding, reduces the number of purification steps needed by combining two procedures in one. The properties of octylamine ligand, allowing for binding of impurities over a broad pH range, high salt concentrations, and in various buffer compositions, enable the use of Capto Core 700 chromatography resin over a wide variety of conditions without impairment of its function. Hence, a sample from a previous purification step can be loaded directly on the Capto Core 700 column, without the need for pre-adjustment of buffer conditions. For our study, these benefits enabled decreased number of buffers needed. The possibility to serially connect Capto Q and Capto Core 700 columns allows for enhanced system utilization and a reduced overall process time.
Before the sterile filtration step, the viral particles were concentrated and transferred into the final buffer by diafiltration using a ReadyToProcess hollow fiber cartridge (RTPUFP-500-C-9S). Finally, the solution was sterilized by filtration using ULTA Pure HC 0.6 μm/0.2 μm sterile filter.
The virus content was determined by measuring the HA concentration by a Biacore method (7). The amount of infectious particles was analyzed by assaying 50% tissue culture infective dose (TCID50). The total amount of virus particles was determined by virus count (Virus Counter 2100, ViroCyt, Denver, CO, USA). Purity was determined by measuring host cell DNA (quantitative PCR), host cell proteins (Biacore method), and total protein (Bradford method).
The results in terms of TCID50, HA yield, host cell genomic DNA-to-HA ratio, and total protein-to-HA ratio are displayed in Figure 5. The infectivity of the virus was retained throughout the process, as shown by the TCID50 titers. A more detailed description of the downstream purification process, virus count and purity analysis is given in the application note 29043549 (5).
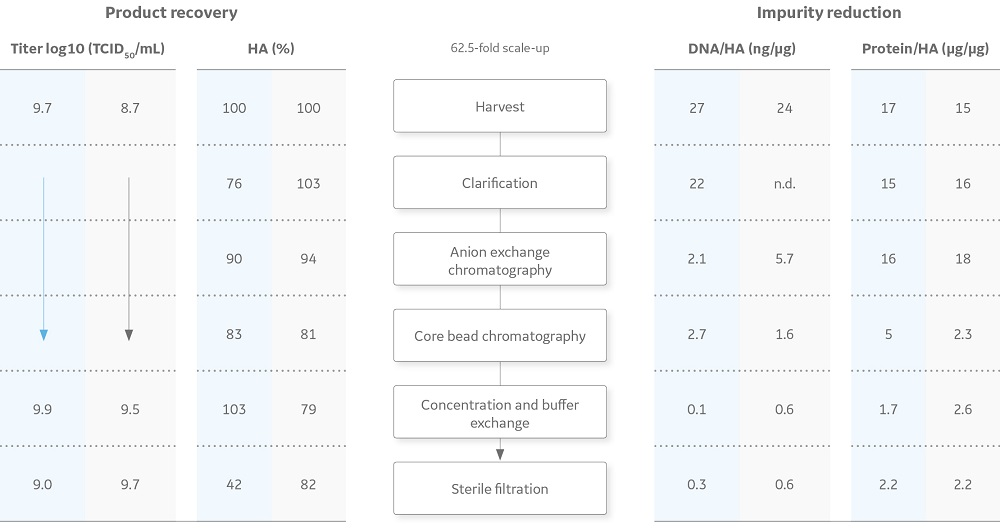
Fig 5. Summary of the outcome from the influenza purification process. Blue values are data derived from the scaled-up production using ReadyToProcess equipment and grey values are data derived from the laboratory-scale production. The overall process time for the downstream purification in the scaled-up production was three days, resulting in a production of influenza virus with comparable yield and purity as obtained in the laboratory-scale production. The genomic DNA-to-HA ratio after clarification in laboratory-scale production was not determined (n.d.).
COMPARISON WITH REGULATORY SPECIFICATIONS
As there is no approved cell-based LAIV on the market today, no regulatory requirements in terms of impurities are established. Hence, the output from the scaled-up production is compared to a commercially available specification for a nasal LAIV and a specification for egg-based, split-inactivated influenza vaccine from WHO (8). The study outcome is summarized in Table 1.
Assuming a nasal route of administration and doses of 107 infectious particles per 0.2 mL dose, the amount of host cell DNA in the scaled-up production was shown to be below the acceptance level (10 ng/dose) defined by WHO. The host cell protein amount per dose and strain in the scaled-up production was also shown to be below acceptance level of WHO. The outcome from the scaled-up production indicates that it is possible to obtain approximately 3000 doses/L harvest, corresponding to harvests of 325 L for 1 million doses (calculations based on specification for nasal LAIV) and 175 doses/L harvest corresponding to harvests of 5760 L for 1 million doses (calculations based on specification for split-inactivated vaccine).
Table 1. Summary of the scaled-up process in doses per liter harvest for the monovalent bulk
| Split-inactivated vaccine* | Nasal LAIV† | |
|---|---|---|
| Scale-up output/L harvest | 175 doses, each 15 μg HA | 3075 doses, each 107 TCID50 units |
| Harvest volume to produce 106 doses | 5760 L | 325 L |
| Protein impurity‡ | 30 μg protein/15 g HA | 1.5 μg protein/107 TCID50 units |
| DNA impurity§ | 3.0 ng/15 μg HA | 0.15 ng/7 TCID50 units |
† Comparison is based on a commercially available specification for a nasal LAIV. A dose of 0.2 mL contains 107 fluorescent focus units, which is assumed to be equal to TCID50 titer.
‡ WHO guideline for protein impurity: max. 100 μg protein/strain
§ WHO guideline for DNA impurity: < 10 ng DNA/dose = 3.3 ng DNA/15 μg HA.
Conclusions
This article describes a scaled-up production of influenza vaccine using ReadyToProcess single-use equipment. Many vaccine production processes are in a scale suitable for single-use equipment and could benefit from the increased flexibility and possibility for optimized facility utilization compared to using traditional stainless steel equipment. Single-use equipment enables quick changeover between products, minimizes risk for cross-contamination between batches, and reduces the need for cleaning and validation operations. This allows for an increase in the annual number of batches and multiproduct manufacturing, with an overall improved process economy as a result.
This case study shows that single-use equipment, including disposable cell culture bioreactors, prepacked chromatography columns, and filters, can replace traditional equipment, including stainless steel bioreactors, user-packed chromatography columns, and ultracentrifuges, for the production of vaccines with high purity.
The case study described in this article is not a fully optimized process. Further optimization of the process is necessary prior to use in vaccine manufacturing.
Read more about our vaccine platforms
References
- Global Action Plan for Influenza Vaccines (GAP). World Health Organization, Geneva. [online] www.who.int/influenza_vaccines_plan/en/ (2011).
- Partridgea, J. and Paule Kienyb, M. Global production capacity of seasonal influenza vaccine in 2011. Vaccine 3, 728-731(2013).
- Pietrzykowski, M., Flanagan, W., Pizzi, V., Brown, A., Sinclair, A., and Monge M. An Environmental Life Cycle Assessment Comparing Single-Use and Conventional Process Technology. BioProcess Int 24, 30-38 (2011).
- Application note: Scale-up of adherent Vero cells grown on Cytodex microcarriers using single-use bioprocessing equipment, Cytiva, 29043548, Edition AB (2014).
- Application note: Downstream scale-up purification of influenza virus using single-use bioprocessing equipment, Cytiva, 29043549, Edition AC (2014).
- Application note: Purification of influenza A/H1N1 using Capto Core 700 Cytiva, 29000334, Edition AB (2012).
- Nilsson, C.E., Abbas, S., Bennemo, M., Larsson, A., Hämäläinen, M.D., and Frostell- Karlsson, A. A novel assay for influenza virus quantification using surface plasmon resonance. Vaccine 28, 759–766 (2010).
- Recommendations for the production and control of influenza vaccine (inactivated). World Health Organization Tech Rep Ser 927:103. (2005).