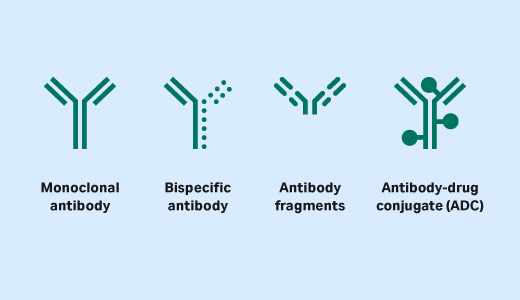
Continuing innovation coupled with demonstrated patient benefit have been driving the design and manufacturing of newer classes of protein-based molecules. Among these novel protein and peptide therapeutics are antibody-drug conjugates (ADCs), multispecific antibodies, antibody fragments, and recombinant and synthetic peptides.
While most approved antibodies are full-length antibodies, market approvals of antibody-derived molecules are on the rise, with many more in clinical trials. These next-generation molecules (Fig 1) offer benefits such as improved efficacy, enhanced selectivity, and lower toxicity compared with available treatment options (1). The structures of these molecules are complex, posing new challenges for both process development and manufacturing.
Fig 1. Illustration of the molecular diversity of antibody derivatives.
Antibody-drug conjugates
One of the most rapidly expanding categories of therapeutic agents in the field of oncology (2). While not a new concept in itself, there has been a wave of innovation around ADCs. However, developing and manufacturing these therapies poses unique challenges. High-potency antibody-drug conjugates require specific upgrades to ensure safety in operator handling. They also require the management of potential exposure to the highly toxic chemicals used to make payload-linker complexes, the ADC drug substance (DS), and drug product (DP).
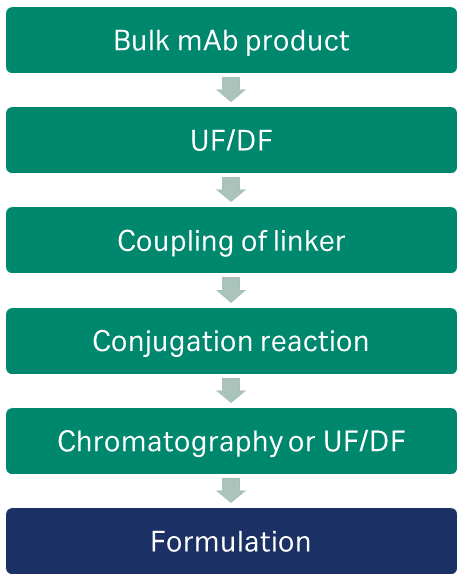
Single-use technologies are beneficial when preparing ADCs, as they can provide a functionally closed and disposable system to protect both operator and product and reduce the risk of cross-contamination between batches. A study to examine extractables in single-use components demonstrated low levels of extractables when exposed to solvents typically used in ADC production. A generic ADC workflow is shown in Figure 2.
Fig 2. Simple workflow for preparing ADC from bulk mAb.
ADC production begins with the manufacture of the antibody intermediate, which necessitates the development of both upstream and downstream manufacturing capabilities. End-to-end solutions, like the FlexFactory™ biomanufacturing process train, provide options at every level of biomanufacturing—process, equipment, platforms, automation, consumables, and services.
Explore these white papers and web pages on ADCs:
- Exploring the world of antibodies: mAbs, Fabs, dAbs, and bsAbs
- A translational perspective: Biacore™ systems in discovery and early-stage development of biotherapeutic antibodies
- Biacore surface plasmon resonance systems in late-stage development and quality control of biotherapeutic drugs
- Simulating chromatography processes with mechanistic modeling
- Cytotoxic drug filling
Multispecific antibodies
Bispecific antibodies are unique in that they can bind to two antigens or to two binding sites on a single antigen. Trispecifics and other multispecific antibodies are also being developed to further extend the number of binding sites. By binding to multiple sites, bispecifics and other multispecifics could enhance efficacy and reduce drug resistance. Bispecifics have been used primarily in oncology applications, but other disease types are being investigated (3). Many structures are possible within the multispecific antibody umbrella, and the complexity makes them challenging to produce and purify. An expanded toolbox of purification solutions, particularly for affinity capture and polishing, can be helpful to address specific needs for these novel molecule types.
Learn more about purification solutions for bispecific antibodies:
- Mechanistic modeling to speed up chromatography process development
- Capture of bsAbs and removal of product-related impurities using a protein L resin
- Optimization of a two-step purification method for bsAbs – collaboration with Alligator Bioscience AB
- Efficient one-step protein A purification of bispecific antibodies
- Sharma P, Joshi RV, Pritchard R, Xu K, Eicher MA. Therapeutic antibodies in medicine. Molecules. 2023 Sep; 28(18): 6438.
- Dumontet C, Reichert JM, Senter PD, et al. Antibody-drug conjugates come of age in oncology. Nat Rev Drug Discov. 2023; 2: 641-661.https://doi.org/10.1038/s41573-023-00709-2.
- US Food and Drug Administration. Bispecific antibodies: an area of research and clinical applications. Last updated August 2, 2023. Accessed January 11, 2024. https://www.fda.gov/drugs/news-events-human-drugs/bispecific-antibodies-area-research-and-clinical-applications.