A guide to cell thawing
The science, research, and methods behind efficient, effective cell therapy thawing.
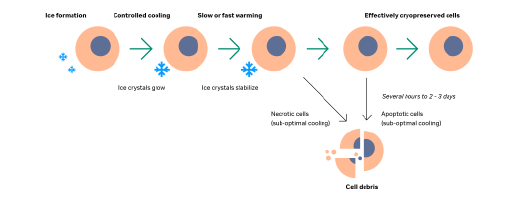
Cryopreservation is an important step in cell therapy manufacturing. All workflows require at least one logistical step, and highly controlled temperature management.
Control product consistency throughout the cryogenic cold chain with Cytiva’s digitally enabled solutions for cryopreservation, logistics, and thawing.
LN2 versus electric technologies — comparison, outcome, and evidence from the ATTC network.