Automation in dissolution testing
Dissolution testing is an essential process in the pharmaceutical industry for evaluating the rate of release of an active pharmaceutical ingredient (API) from its dosage form. Automated solutions for this process help laboratories improve accuracy, precision, and productivity.
Why automate dissolution testing?
Dissolution testing can be performed manually or using semi- and fully automated systems. In all cases, it is necessary to comply with specifications outlined in the relevant pharmacopeia. It’s also a good idea to compare manual and automated procedures to evaluate the interchangeability of the procedures.
As dissolution testing requires multiple sampling and sample preparation steps, automation can improve efficiency by significantly reducing the amount of manual labor involved, Automation might be used for:
- Preparation and dispensing of dissolution media
- Introduction of the dosage form into the dissolution media
- Sampling
- Preparation of the sample for analysis
- Data collection and analysis
As the sampling must be implemented at specified time points, automating all or part of the workflow minimizes variables that might otherwise contribute to out-of-specification results. The US Pharmacopeia (USP) General Chapter 11 (2020) specifies that sampling and filtration must occur within ± 2% of the stated time point. For example, a 10-minute timepoint requires pulling and filtering a sample within 12 seconds of the intended time, something that is only reliably achievable through automation.
Filtration in sampling
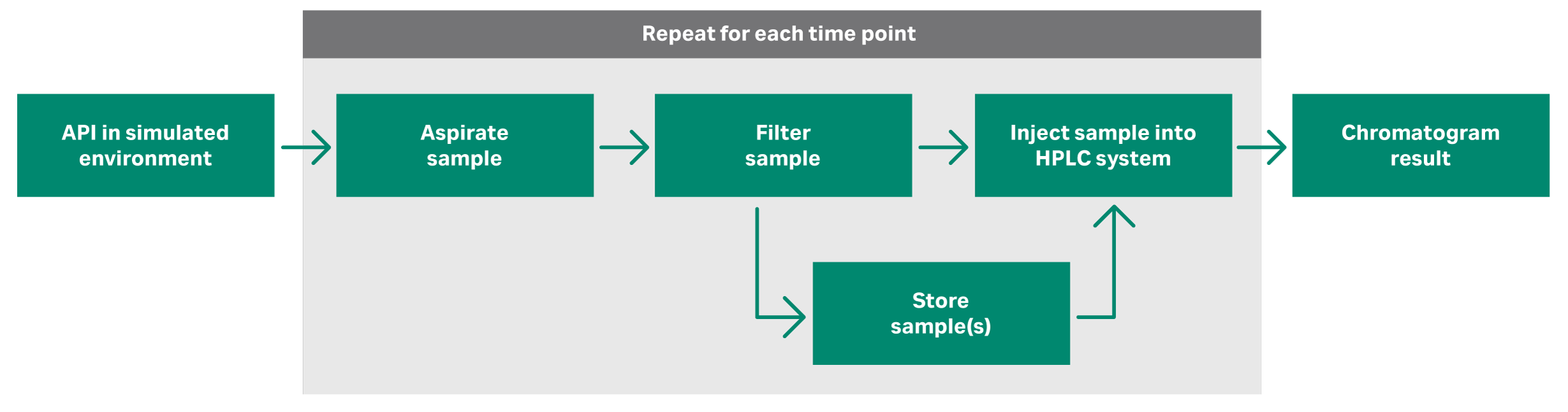
Autosamplers can help maximize data consistency in dissolution testing. As the name suggests, autosamplers automatically draw samples from the dissolution vessel at specified time points. These instruments then prepare samples for collection or injection into analytical instruments like UV-Vis or high-performance liquid chromatography (HPLC) systems.
Filtration plays a key role in sampling for dissolution testing. The selection and use of filters in the workflow can affect data consistency and accuracy. Filtration of the sample, either through cannula filters at the tip of sample probes, glass filter circles or via in-line syringe-type filters, halts the dissolution process and separates any undissolved active pharmaceutical ingredient (API) and excipients.
Filtration enables samples to be stored for analysis, or immediately injected into the analytical instrument without introducing particulates that could damage or block the column frit.
Fig 1. A typical dissolution sampling workflow.
Selection and use of filters
Several factors contribute to the effectiveness of sample filtration and subsequent accuracy and consistency of downstream analyses. Whether using cannula, glass filter disc or syringe-type filtration device, a filter can vary in its suitability for a given drug formulation and dosage form.
Our syringe filters are compatible with dissolution systems including the Roby filter, with the polypropylene housing designed to ensure smooth transport from the storage turntable to the filtration site. Additionally, we offer the Whatman™ 850-DS 8-channel filter plate, exclusively designed for use with the optional filter module on the Agilent 850-DS dissolution sampling station to simplify filter replacement between timepoints.
If a filter device is used for more than one time point for the same sample, flushing will not be necessary at every time point. Validation tests can identify the sample volume that needs to be flushed and the number of samples that can be filtered before clogging. These tests enable analysts to set up an autosampler to flush and replace filters automatically according to the standard operating procedure (SOP).
Filter selection influences the accuracy and reliability of dissolution data. Read our article on Filtration in dissolution testing to learn about the factors that influence filter selection and membrane options for dissolution testing.
Try our Whatman™ Filter Selector App to find the most appropriate filtration solution for your samples based on the properties of your API and dissolution media. To discuss any challenges you are facing, please contact Cytiva Scientific Support.