Surface plasmon resonance (SPR) assays are used across the life cycle of a biopharmaceutical, from target identification, through CQA determination, development, and on-going quality control. This article focuses on concentration assays associated with late-stage development and biotherapeutic drug chemical manufacturing and control.
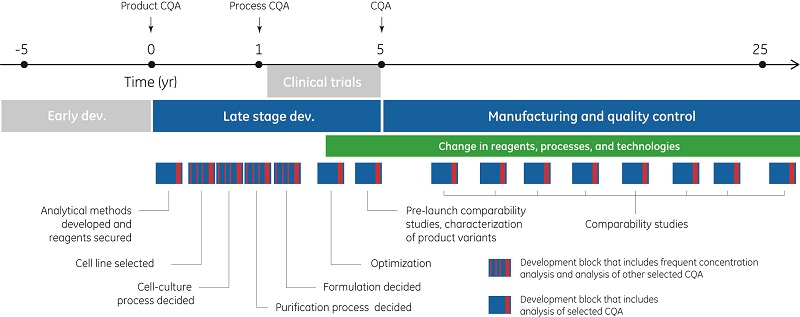
The life cycle of a biotherapeutic product typically spans over thirty years. Early development can exceed five years, followed by late-stage development and clinical trials. Once approved for an initial indication, a successful product can remain in manufacturing and quality control for over twenty years, with additional indications added over time.
The growing trend of biosimilars, more complex antibody therapeutics and biologics puts increasingly higher demands on the assays and technologies used for screening, characterization, comparability and release testing. Attrition of therapeutic candidates during clinical development is the major factor in high development costs. Developability assessment teams are charged with evaluating potential drug candidates to allow pharmacologically effective new biological entities with favorable toxicity, immunogenicity and efficacy profiles to proceed through development, and decrease the risks of late-stage attrition. As the lead candidate enters late-stage development, it is important to define early on a detailed quality profile that is based on desired biological outcome and function, i.e. mode of action.
This comprehensive characterization of the candidate molecule is then used to set the appropriate range of critical quality attributes (CQA). These characteristics should be evaluated using the most sensitive approaches to identify drug product differences and evaluating the biological functions which are central to the mechanism of action. Surface plasmon resonance (SPR) using Biacore systems is widely used across the biopharma industry for both characterization and quality control.
Critical quality attributes are fundamental to regulatory compliance
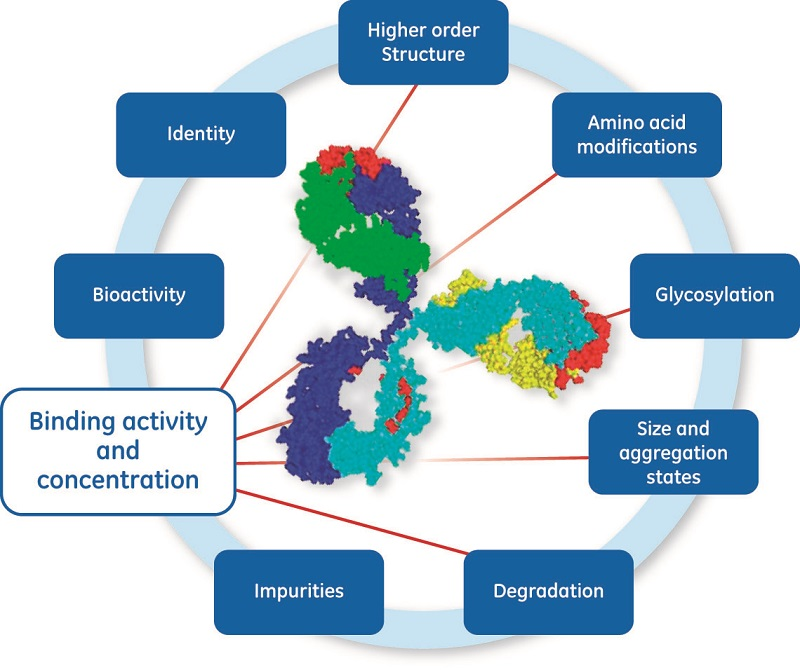
CQA typically include data on how the candidate interacts with target proteins, as well as process-related CQA such as protein integrity, homogeneity, presence of host cell proteins, host cell DNA, and substances released from process or package material. (Fig 1) For each step in the process, critical process parameters (CPP) that can affect CQA are identified (1) and a control strategy is defined. To support this process, requirements for test procedures and acceptance criteria have been described in regulatory guidelines (2) for biotechnological/biological products.
The quality, accuracy and reliability of assays is particularly critical when developing biologics and biosimilars, and the functionality of the target molecule is key for development of the panel of assays as illustrated in Figure 2 required to ensure drug product quality and process consistency across the life cycle. A broad range (50 to 60 variants) of different analytical technologies may be used for CQA analysis (3). Enzyme-linked immunosorbent assay (ELISA) and surface plasmon resonance (SPR) are commonly used for in vitro confirmation of biological activity by measuring interactions with antigen, receptor, FcR, or other binding proteins (3, 4). This article focuses on concentration assays associated with late-stage development and biotherapeutic drug chemical manufacturing and control, CMC (5).
During process development, the goals are to obtain high product yield and good process economy to ensure that properties related to initial CQA are maintained and to secure that the process itself is well controlled with low impurity levels. Throughout all steps of late-stage development, initial CQA are monitored and possible new CQA are evaluated. Before transfer to manufacturing, the complete list of CQA is established along with methods for their control. Manufacturing and quality control (QC) ensures a supply of product with consistent quality to the market. CQA are determined using state-of the-art knowledge at a given time and might later have to be revised based on clinical data that accumulate over time. Regulatory authorities impose increasing demands on pharmaceutical development and manufacturing companies to use quality-assured equipment and to follow detailed and carefully documented analytical and manufacturing procedures. The purpose of this control effort is to ensure consistent and reliable quality in pharmaceutical products that reach the market.
Biacore assays are based on ligand binding
Ligand-binding assays are key for characterization of biotherapeutic medicines. SPR and ELISA are extensively-used ligand-binding assays. Biacore systems and ligand- binding assays from Cytiva’s business are based on surface plasmon resonance (SPR) analysis, and have been used for antibody characterization for more than twenty years.
The readout from a Biacore system is related to molecular mass, and any binding event can be detected without the use of labels. The readout is continuous, which allows quality control of the entire binding event. This provides opportunities for data analysis based on binding responses obtained at one or several specific time points (report point analysis) or by comparing and even fitting entire binding curves for determination of kinetic, affinity and comparability to reference product.
Biacore systems measure active concentration with high precision
Concentration analysis is vital in several process development steps: to determine the yield in cell cultures over time; for identification of suitable chromatography conditions; for monitoring of final purification processes; and in formulation studies.
Concentration measurements in their simplest form can be based on values of absorbance measured at 280 nm wavelength (A280 values), but this requires that interference from other UV-absorbing substances is ignored. A280 values are often used in combination with HPLC studies. It can, however, be of more interest to produce concentration data that are related not only to total protein concentration but to the specific protein and even to the function/activity of the protein.
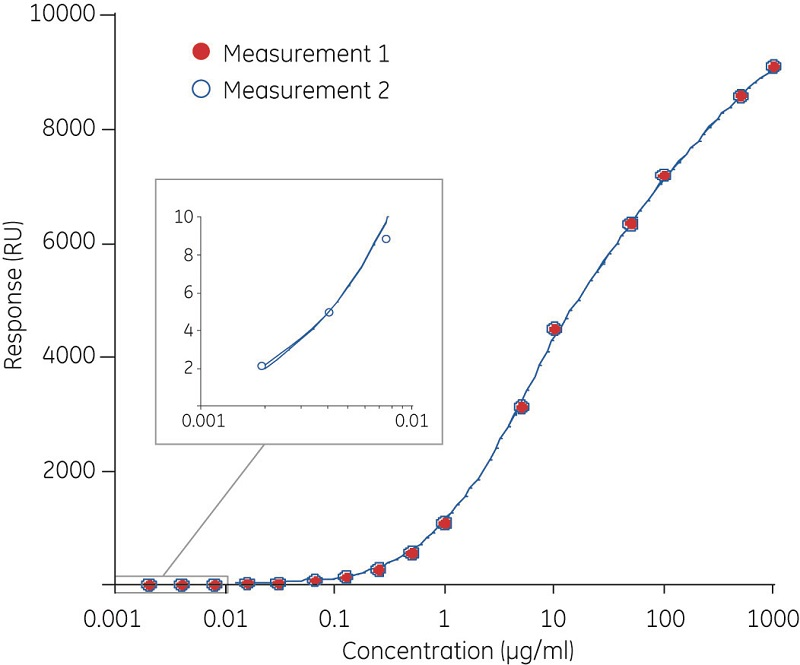
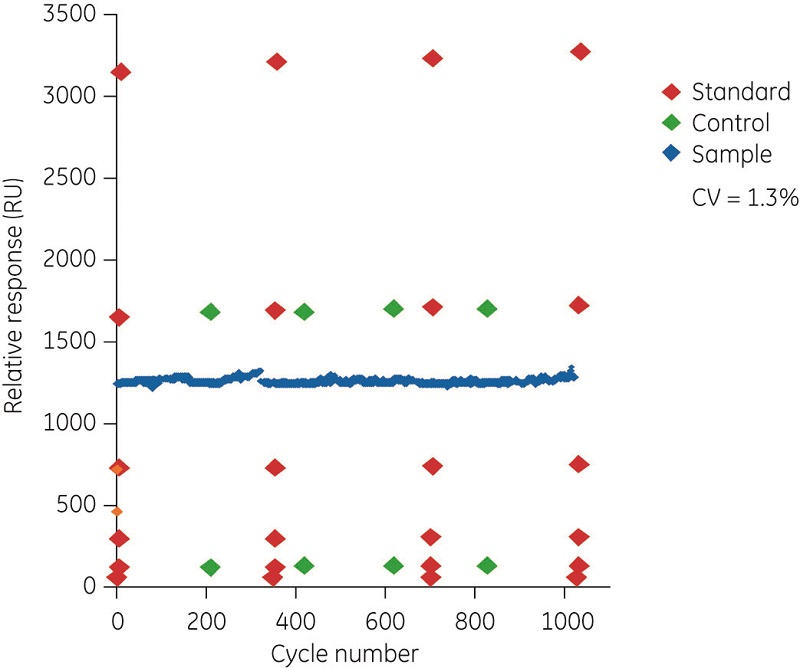
Figure 3 illustrates the dynamic range and precision in concentration analyses using Biacore systems. The assay readout is from a report point at the end of sample injection. In these examples, an antihuman Fc antibody is immobilized on the sensor surface. In Figure 3A, omalizumab at concentrations from 2 ng/ml to 1 mg/ml was injected for a 3 min period. The insert shows the response for the lowest (2 to 8 ng/ml) concentrations. This assay can be fine-tuned and, depending on the concentration range of interest, the analysis time can be adjusted. In Figure 3B, the sample injection time was reduced to 20 s and the concentration range of interest was from 0.5 to 50 µg/ml. The figure shows data obtained with repeated injections of standards, controls, and one selected sample over 1000 analytical cycles obtained on the same surface. Noticeably, standard and control samples were stable over time and the coefficient of variation for the repeat sample was 1.3%.
Clearly, Biacore assays are sensitive, have potential for a wide dynamic range, can be very rapid and, based on the Biacore system design, can be multiplexed with the possibility to run several assays in parallel.
Novel calibration-free concentration analysis for rapid concentration measurements
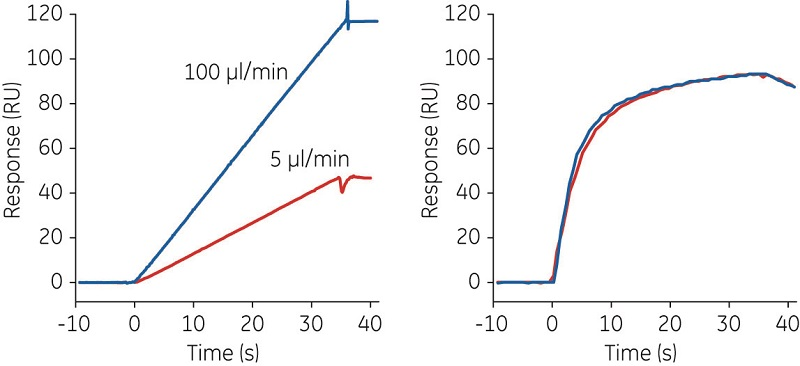
Concentration analysis based on ligand binding typically requires a standard preparation with known active concentration. However, a standard preparation might not always be available, for example, when a protein is expressed for the first time or when concentration data for the standard reflects the total protein concentration and not the active concentration. In such circumstances, Biacore analysis can be used for direct assessment of the active concentration. This possibility was first described in 1993 (6) and has been refined with modern numerical integration tools for data analysis (7). The calibration-free concentration analysis (CFCA) method illustrated in Figure 4 provides a good estimate of the absolute concentration, assuming that the diffusion coefficient of the analyte is known and that the observed binding rate is flow-rate dependent.
Multiple uses of CFCA
CFCA is an excellent tool for comparison of concentration data and is particularly useful for reagent characterization. CFCA is also a complement to kinetic analysis and sensorgram comparison methods when these indicate that changes in the interaction can be related to changes in active concentration. The use of CFCA for analysis of chromatography fractions and to guide purification efforts is described in Reference 8. Considering that relative concentration data is so easy to obtain with CFCA, it may also be used to complement and support potency assays.
Summary
The life cycle of a biotherapeutic agent can extend over twenty to thirty years. During this time, a manufacturer must develop and deliver a product with consistent quality. In support, regulatory authorities such as FDA and EMA issue guidelines aimed at ensuring efficacy of biotherapeutic medicines. Biacore systems have been developed to function in a regulated environment and come with installation and maintenance support and with software that combines flexibility in development with rigor and reproducibility in a QC environment.
SPR, a direct-binding technique, is relevant to a broad range of applications throughout the biotherapeutics life cycle. SPR using Biacore systems is used in early development to define CQA. In late-stage development, SPR can be used for concentration analysis in cell culture, purification and formulation workflows, while concentration assays with Biacore are a useful tool for quality control throughout the manufacturing phase. The CFCA method introduces novel opportunities for rapid absolute and relative concentration measurements, with implications for potency assays.
For a more comprehensive discussion of the many applications of ligand-binding assays with Biacore system, see the white paper, Biacore concentration and ligand-binding analyses in late-stage development and quality control of biotherapeutics.
- Mitchell, M. Determining criticality-process parameters and quality attributes. Part I: Criticality as a continuum. BioPharm International 27, 38–47 (2013).
- European Medicines Agency ICH Topic Q 6 B. Specifications: Test Procedures and Acceptance Criteria for Biotechnological/Biological Products. (2006).
- Lubiniecki, M. F. A. et al. Analytical lessons learned from selected therapeutic protein drug comparability studies. Biologicals 41, 131–147 (2013).
- Visser J. et al. Physicochemical and functional comparability between the proposed biosimilar rituximab GP2013 and originator rituximab. BioDrugs, 27(5), 495–507 (2013).
- Jones, S. D. et al. CMC activities for Development of Mabs, Critical steps to reach IND with a therapeutic antibody. Contract Pharma, pp 60–63 (20I0).
- Karlsson, R. et al. Analysis of active antibody concentration. Separation of affinity and concentration parameters. J. Immunol. Methods 166(1), 75–84 (1993).
- Pol, E. The importance of correct protein concentration for kinetics and affinity determination in structure-function analysis. J. Vis. Exp. 17, 1746 (2010).
- Application note: Accurate comparability assessment of a biosimilar interferon in process development, Cytiva, 29-1154-78, Edition AC (2014).