A high-resolution ion exchange chromatography run is key for obtaining high protein purity. The better the separation between peaks, the higher the purity of the proteins that will be collected. Here’s a handy check list of IEX tips and tricks for obtaining well-separated peaks on your chromatogram. For detailed recommendations, full article is available under the table.
| Your checklist to optimize ion exchange chromatography (IEX) resolution | ||
|---|---|---|
| IEX running conditions |
|
|
| IEX column maintenance |
|
|
| IEX resin |
|
|
| System configuration |
|
|
| Multistep purification |
|
|
IEX running conditions
The easiest way to improve the resolution from an IEX run is to modify the running conditions. Different pH will affect the charged surface on the protein, which affects the resolution. A smaller amount of sample will improve the resolution: typically use up to 30% of the complete capacity to maintain good resolution. Reduced flow rate will also improve the resolution.
Decreasing the sample load will increase resolution
The amount of sample which can be applied to a column depends on the protein binding capacity of the chromatography resin, and the degree of resolution required.
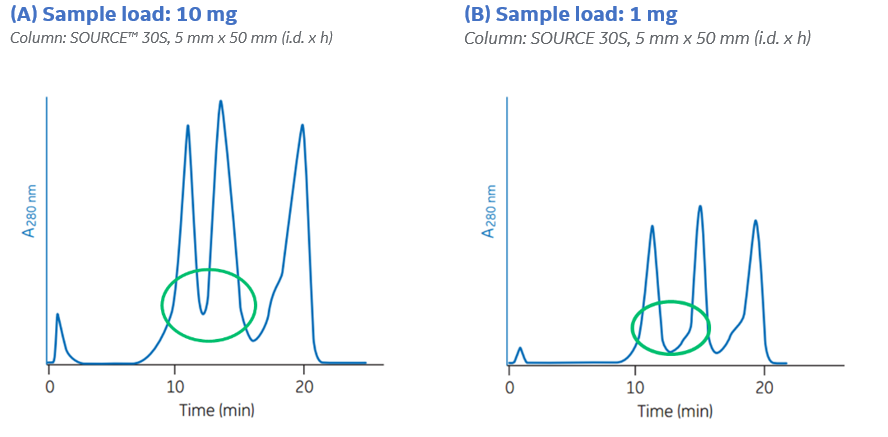
If the IEX resolution is unsatisfactory, you can try to decrease the sample load. Figure 1 below shows two runs on the same IEX column where sample load was different.
A rule of thumb is to never apply more than 30% of the total binding capacity of the chromatography column for optimal resolution using gradient elution.
Fig 1. Decreased sample load typically improves the resolution. By decreasing the load from 10 mg (A) to 1 mg (B), the resolution between the two first peaks was increased, as indicated by the green circles.
Decreasing the flow rate will increase resolution
Flow rate during the elution phase can have an impact on the IEX resolution.
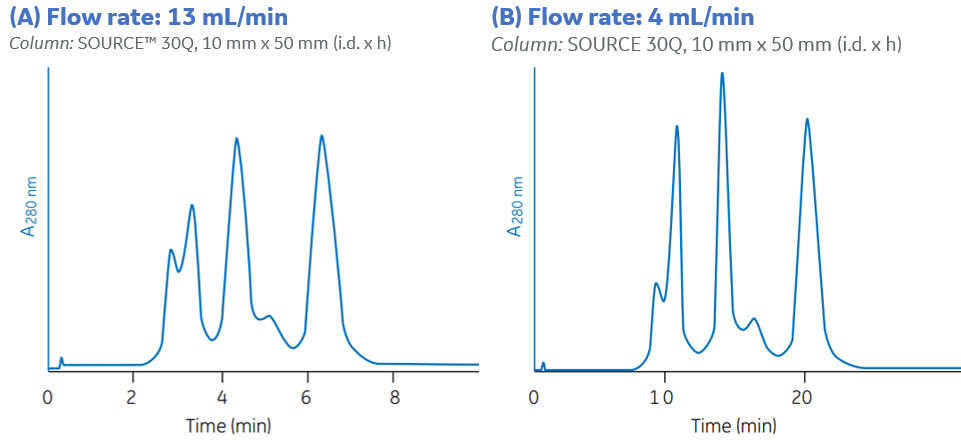
The chromatograms below show two runs on the same IEX column where the elution of the proteins was done at different flow rates. When decreasing the flow rate from 13 mL/min to 4 mL/min, the separation between the proteins was increased, as can be seen in Figure 2(B).
Fig 2. Decreased flow rate typically improves the resolution, here from 13mL/min (A) to 4 mL(min (B)
A shallower elution gradient will increase resolution
Optimization of the elution buffer application method can also improve protein purity. A shallow gradient slope typically improves separation.
Linear gradient elution is most commonly used in small scale IEX applications. A good starting point is to use a linear salt gradient from 0 to 1 M NaCl during a volume corresponding to 10–20 column volumes (CV).
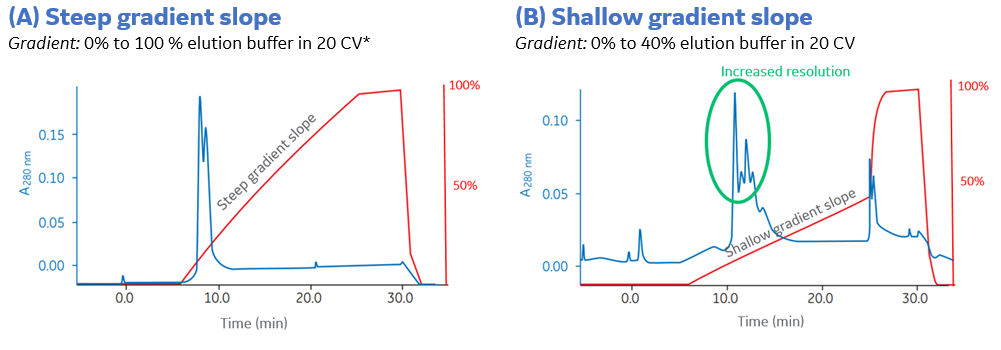
The slope of the elution gradient influences the resolution where a shallower gradient typically improves the resolution, as shown in Figure 3. The increased resolution is indicated in green (Fig 3B).
Fig 3. The shallower gradient increases the resolution indicated in the green circle in the right chromatogram. *CV = column volume. (A) The first purification run was performed with a gradient 0-100 % elution buffer during 20 column volumes (CV), a good starting point for a small scale IEX separation. Two peaks elute in the beginning of the gradient, up to ~30% elution buffer. (B) To improve the resolution a shallower gradient is applied in this area, a gradient from 0 to 40% elution buffer during 20 CVs. The shallower gradient during the elution achieved an increased resolution, as indicated in green.
Back to checklist
IEX column maintenance: a clean IEX column improves resolution
It is important to keep the column clean to maintain IEX column performance. Without column maintenance, impurities can adhere to the beads and negatively affect the IEX resolution.
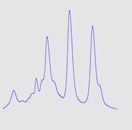
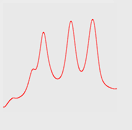
Figure 4 shows the resolution between standard proteins using a 1 mL IEX column with a fresh column (A), after purification with 30m E. coli lysate (B) and after cleaning (C).
As can be seen, almost half of the resolution was lost after purification with 30 mL E. coli lysate, shown in the middle in Figure 4.
By cleaning the column with 1 M NaOH (3 x 2 mL), the resolution was restored, as shown in Figure 4 (C).
| (A) Fresh column: | (B) After a purification step using 30 mL E. coli lysate as sample: | (C) After cleaning the column with 1 M NaOH (3 x 2 mL): |
|---|---|---|
 |
 |
 |
 |
 |
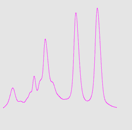 |
Fig 4: Resolution for standard proteins on a fresh column (A), after injection of 30 mL E. coli lysate (B) and after cleaning (C). Top row shows microscope pictures of the beads in the column; bottom row shows chromatograms. Column: HiTrap™ Q HP 1 mL. Sample: standard proteins.
Back to checklist
IEX resin
Resin and column parameters will influence your IEX resolution as outlined below. In addition, to help you select the most suitable IEX resin or IEX column to purify your precious protein sample, see our online selection guide.
Smaller resin particle size will improve resolution
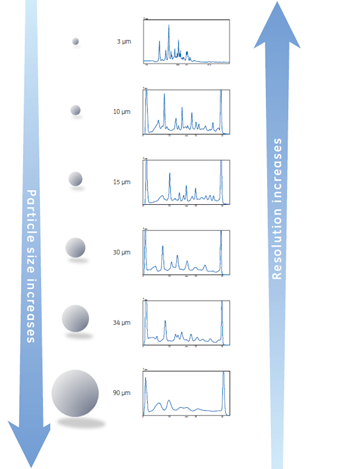
As shown in Figure 5, a smaller chromatography bead can increase the resolution since it improves the efficiency of the column.
On the other hand, be aware that smaller particles often create an increase in back pressure and can cause column bed compression and column leakage, as well as breakage of system components if the flow rate is not decreased. Therefore, smaller particles give higher resolution, but the run times will be longer. So, the choice of the resin particle size will depend on your priorities: either resolution, or high flow rate, or an acceptable compromise of both.
Fig 5. Examples of the influence of particle size on the final resolution.
Fig 6. Two columns with the same chromatography resin and volume but different dimensions will give different resolution and different pressure.
Back to checklist
Prevent IEX resolution loss with correct chromatography system configuration
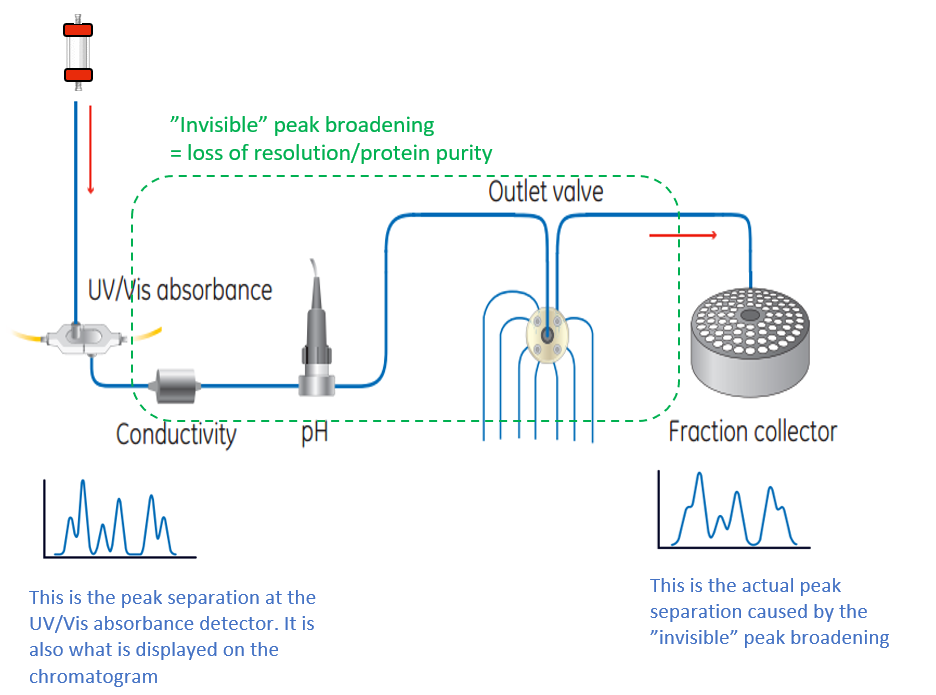
About peak broadening
The resolution seen from the UV detector is not always the same as the one obtained in the fraction collector, since diffusion of the peaks occurs during the transportation from the UV detector to the fraction collector. This is called peak broadening.
Note that narrow, high resolution peaks are significantly affected by the chromatography system contribution, whereas wide, more preparative peaks are relatively unaffected by the system contribution. For example, in most cases, HiTrap columns are unaffected by the system contribution, whereas high resolution columns such as MiniBeads and MonoBeads are more affected.
Fig 7. The resolution seen in the UV detector is affected by diffusion of the peaks, called peak broadening, during the transportation to the fraction collector. Minimize the volume (components and tubing) between the UV cell to maintain the resolution all the way to the fraction collector.
How to minimize peak broadening
To minimize peak broadening (hence maintain high resolution), it is important to keep the volume between the column and the fraction collector as small as possible.
- Use short tubing lengths between all components.
- Remove unnecessary components.
- Use capillaries with a low inner diameter.
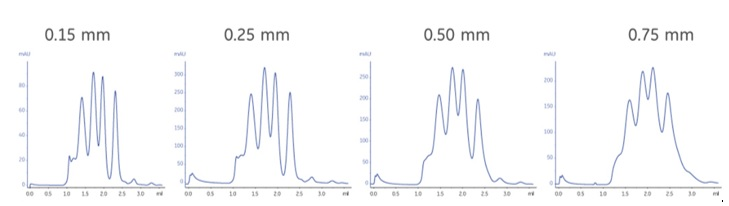
Fig 8. Effect of tubing diameters on resolution using a specific column and mix of standard proteins
Back to checklist
Multistep purification: add a SEC step after IEX to improve the protein purity
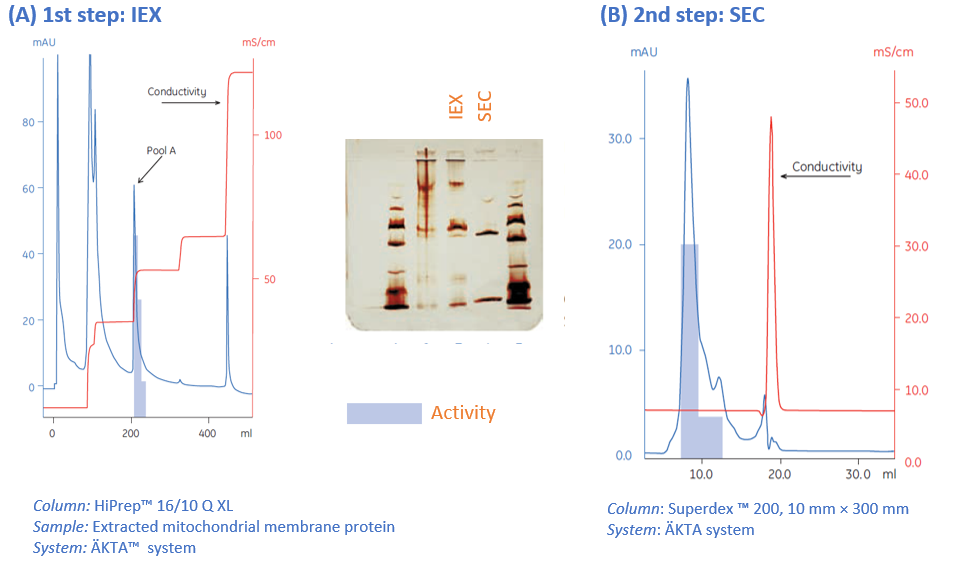
Combining different techniques in your protein purification protocol is a common way to get the level of purity needed. Typically, to further improve purity after IEX, a polishing size exclusion chromatography (SEC) step can be added, where the proteins are separated according to size (Fig 9).
Explore how to best combine IEX technique with other chromatography techniques to obtain the required level of purity and yield.
Fig 9. An additional size exclusion step was added after the first ion exchange step to improve the purity. (A) The chromatogram of the ion exchange run. Analysis of the eluted fractions shows that the activity of the protein was found in three fractions. (B) These fractions were pooled, and 0.4 mL was then applied onto a size exclusion column. The results show that most of the activity was found in the main peak.