This article describes how to combine chromatography techniques to optimize your antibody purification protocol and obtain the right balance between antibody (immunoglobulin) purity and yield. It describes proven technique combinations for the purification of antibodies.
Antibody purification, purity and yield: get the balance right
Antibody purification protocols are typically challenged by two factors:
- Capturing as many antibodies as possible without degrading the sample.
- Removing the remaining impurities and minimizing aggregate content.
With that in mind, what should be considered when planning your antibody purification experiment?
- The choice of chromatography techniques depends on the purity requirement of your antibody of interest. The required antibody purity level will depend on your application, as shown on the table below.
- Before you start, carefully define your objectives and consider that in general, every added purification step will increase purity but decrease total protein recovery1 and yield2.
| Typical applications | Purity level |
|---|---|
| Mass spectrometry Antigen for immunization | Moderate to high, 80% to 90% |
| Functional studies Structural studies | Very high, 95% to 99% |
| Structural studies Therapeutic proteins | Highest > 99% |
1 Recovery = The relative amount of target protein (%) that is retrieved after purification compared with amount loaded on the column.
2 Yield = Amount of target protein obtained after a purification step, or after the entire purification (multiple steps).
What do antibody purification protocols look like?
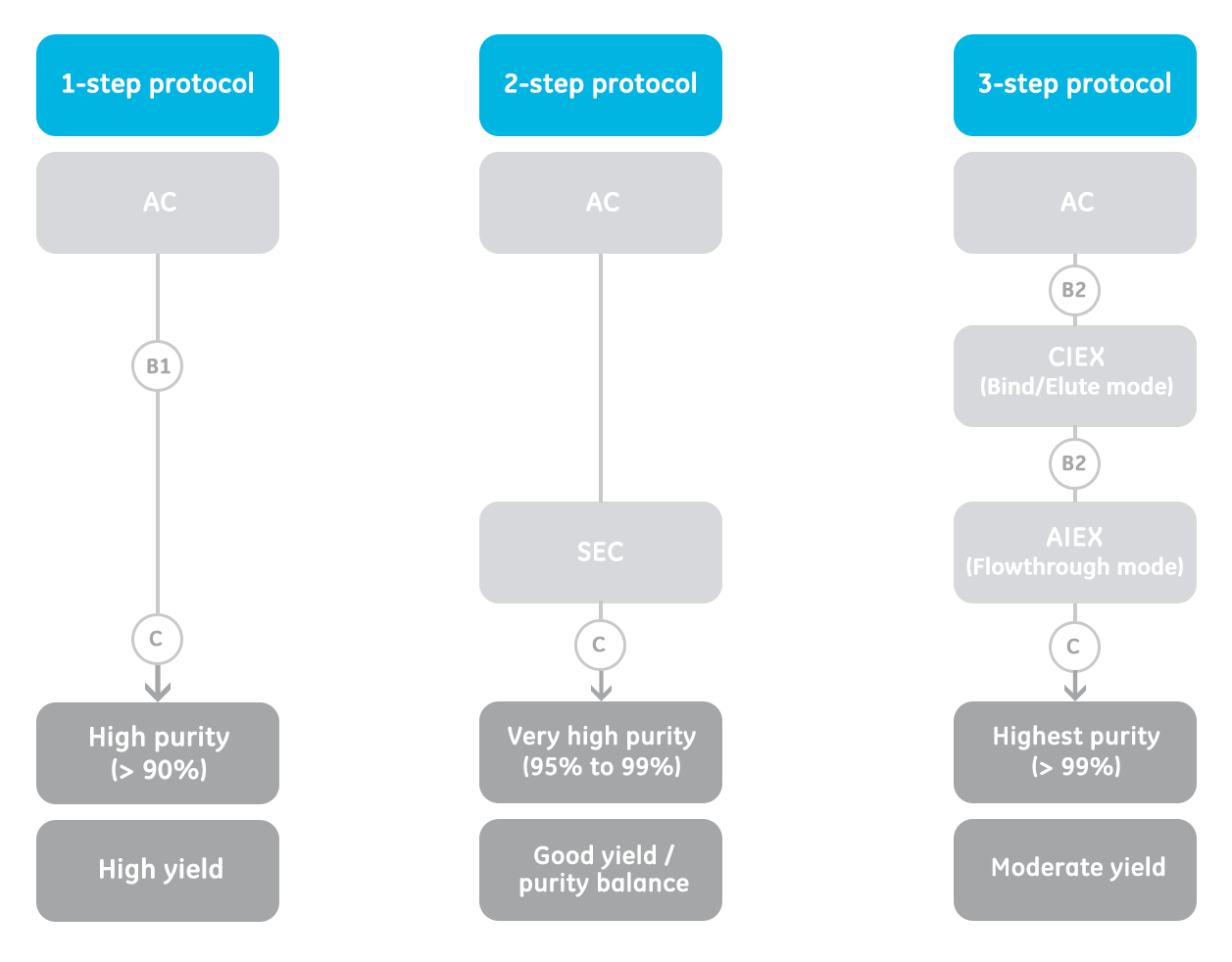
Figure 1 describes typical proven technique combinations for the purification of antibodies.
All antibody purification protocols typically start with an affinity chromatography step (AC).
It enables the isolation of antibodies from initial sample (e.g., serum, cell culture)
1-step protocol and 2-step protocol:
The 1- and 2-step protocols are the recommended best choices for research use.After the AC step, the purity level is usually high (> 90%). However, if there is a need to remove antibody aggregates and/or fragments to obtain monomeric antibodies, then a size exclusion chromatography (SEC) step is recommended. The purity level after a 2-step purification will be very high (95% to 99%).
3-step protocol:
Consider using the 3-step protocol for scale-up or process development. SEC is not used as a final step to remove aggregates, fragments, or other impurities, due to the limitation of sample volume. Instead, a combination of IEX (ion exchange chromatography) steps is used.
After the AC step, cation exchange chromatography (CIEX) is first used in a "bind elute" mode to further remove HCP leached protein A ligand, mAb aggregates, fragments, and other isoforms.
Then the sample is applied to AIEX (anion exchange chromatography) column in a "flowthrough" mode for a final removal of remaining impurities of HCP, DNA, and viruses.
The antibody purity will then be extremely high (>99%).
Importance of buffer exchange and concentration in the antibody purification protocol
To make the sample compatible with the following steps or experiments, it might be necessary to use a desalting column for buffer exchange, and/or a concentration unit to reduce the sample volume.
Which chromatography columns are recommended for each step?
Click on the blue buttons to get recommendations on the most suitable columns for each step.
Recommended systems:
ÄKTA start
ÄKTA pure
ÄKTA start
ÄKTA pure
Recommended chromatography systems:
ÄKTA avant
HiTrap Protein A HP
HiTrap Protein G HP
HiTrap MabSelect PrismA
HiTrap MabSelect SuRe
HiTrap Protein A HP
HiTrap Protein G HP
HiTrap MabSelect PrismA
HiTrap MabSelect SuRe
HiTrap MabSelect PrismA
HiTrap MabSelect SuRe
HiScreen MabSelect PrismA
HiScreen MabSelect SuRe
HiTrap Capto S ImpAct
HiScreen Capto S ImpAct
Superdex 200 Increase
HiLoad Superdex 200 pg
HiPrep Sephacryl S-300 HR
HiTrap Capto Q
HiScreen Capto Q
Fig 1. 1-, 2-, or 3-step protocols may be deployed to purify antibodies depending on the goal of the purification—high yield, good yield/good purity, or high purity. AC = affinity chromatography; SEC = size exclusion chromatography; CIEX = cation exchange chromatography; AIEX = anion exchange chromatography. Steps in circles are optional and are applied if necessary.
- B1: Buffer exchange to neutralize low pH Ab elution buffer.
- B2: Buffer exchange to prepare for IEX.
- C: Concentration for sample volume reduction. May also be performed before SEC.
Automate the multistep purification protocol to save time and add capacity
Some AC purification protocols can be performed with a syringe or a pump during the purification. However, for speeding up purification, and for multistep purification, consider upgrading to a chromatography system.
Read how to automate multistep purification with ÄKTA systems.