Maximize control. Minimize risk.
How to Adapt Your Existing Biomanufacturing Process To Maximize Production
As the need for more complex molecules drives a new era of biomanufacturing, there is an increased focus on developing processes with improved security, yield, and process economy. Companies working with legacy equipment face the decision of keeping their existing setup and process or adding modernized technologies to boost efficiency. There may be a perception that there are too many factors to consider when implementing these changes, which could range from simply a change of a single-unit operation to the majority of the process.
The key to adapting existing biomanufacturing processes and creating “smarter” workflows is not just knowing what options are available for doing so. It is also being able to select and successfully implement the ones that will offer the most flexibility while still controlling costs and maintaining quality. However, achieving these goals requires a complete understanding of these novel solutions and technologies, which, when applied appropriately, can push the boundaries of drug development and offer critical advantages in an increasingly competitive industry.
Do You Have An Intelligent Process?
Innovation is on the rise in biopharma in both the molecules pursued and the facilities and equipment used to develop them. Several factors, including demand uncertainty, cost pressures, and speed to market, are driving companies to seek solutions that offer more flexibility and responsiveness in biomanufacturing. This requires selecting equipment that creates the most appropriate unit operations and then making sure they are connected through tools that increase monitoring and control, such as automation. An intelligent process can ensure the operations stay within the desired parameters and adjust to any variations before they become problems.
Once the system is connected, you can then add analytical capabilities. Now, instead of having to do, for example, an offline sampling and then a microbial or product release on the sample, this step can be integrated into the workflow itself using sensors that can detect, for example, glucose and viable cell density. The glucose sensor can trigger an addition through the feed pump, which increases cell density and product titer. In addition, rather than taking manual samples to measure offline for glucose concentration, thereby risking contamination, a viable cell density sensor can react to the cell density increase by bleeding the cells in order to maintain the target cell density. A glucose feed control loop can also be connected to the VCD sensor output.
Intelligence has also been integrated into the control strategy by integrating external scales with internal pumps that communicate and control by the scales. So, once a vessel on a scale achieves a specific weight, the pump reacts by starting or stopping as appropriate to achieve the set point weight.
Other opportunities are now possible, such as real-time product release or built-in feedback or feed forward control, so each step is executed quickly and efficiently without manual intervention. In designing such a process, it eliminates non-value adding activities associated with a typical biomanufacturing workflow, such as buffer preparation. Cytiva's inline conditioning system, BioProcess™ IC System, does this by allowing on-demand production of buffers from single-component, highly concentrated stock solutions of salt, acid, and base, diluted with water. It is designed to manage buffers efficiently, overcoming the buffer bottleneck and large process liquid volumes and saving both time and space. Accuracy is then ensured through dynamic control using different modes of feedback control.
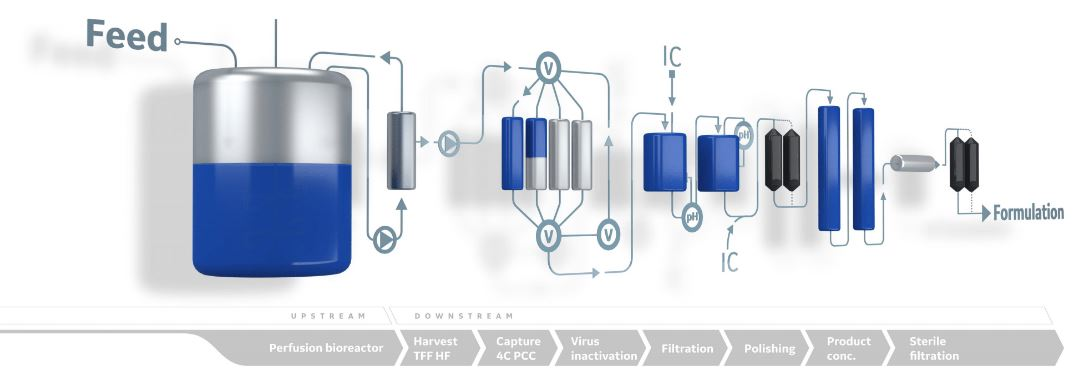
Figure 1: Designing a biomanufacturing process with inline conditioning (IC), automated buffer preparation technology can lead to a more efficient use of existing resources, including labor and consumable savings, and smaller facility footprints.
Plug-and-play options that reduce the need for human intervention can help offset concerns companies may have about whether they have the workforce necessary to implement new technologies. For example, chromatography columns are traditionally packed by using either slow packing or pack-in-place technology that requires skilled operators. Now, Cytiva offers automated chromatography columns that use preprogrammed packing methods to ensure reproducible performance and intuitive handling that guides the operator through key steps. The AxiChrom columns includes an Intelligent Packing feature that delivers verified methods and simplified workflows that can decrease packing time by up to 80 percent.
The cost of column preparation, packing, and validation procedures can also be eliminated with Cytiva’s ReadyToProcess single-use, prepacked chromatography columns, which are available with a wide range of resins. Their design and validated automated packing methods enable consistent column performance and offer customer safety stock possibilities that drive security of supply. Purification processes are scalable between the ReadyToProcess and AxiChrom column formats, which is a key factor in meeting product demand.
Expanding Scalability
Modern technologies available today offer new opportunities to improve process efficiency, increase flexibility, and lower the cost of goods in biomanufacturing. Yet, making sure what you do at a smaller scale can easily be carried over to a large scale is essential. That is why the system you use should give you the confidence that, once you have done something at lab scale, it can be easily implemented at a larger scale without requiring rework that introduces risk and uses valuable resources.
For example, with well-controlled software, reliable hardware, and adaptable consumables, you can accommodate a wide variety of cell types as well as modes of cultivation for perfusion versus batch. Cytiva’s Xcellerex XDR single-use bioreactor systems provide scalable and robust stirred-tank performance for up to 2,000 liters in both cGMP and non-cGMP environments. It has many options for connections to all potential associated operations, including feeds, pH control, perfusion, and downstream operations. This offers a scalable solution regardless of what phase of development you are currently in. Cytiva’s WAVE 25 bioreactor offers controlled perfusion in a closed, single-use bioreactor that makes it possible to go from seed train to full production in a single step, cutting the complexity of your cell culture expansion process. Using a perfusion filter, the bioreactor produces higher biomass in a very small footprint by adding to your production reactor. An N-1 culture at 25 L scale can now be used to seed a production culture with a final volume of 2000 L.
In addition, employing strategies that ensure your downstream processes can keep up with upstream production. These include easy scale-up of chromatography steps, higher-capacity chromatography resins and systems, and shorter process times. Cytiva chromatography systems typically have a wide working range that enables usage across a scale of applications, including process development and pilot scales. This facilitates easy transfer of process development constructs to manufacturing-scale.
Cytiva also has a chromatography system, ÄKTA pcc designed for continuous chromatography at process-development scale that enables scalable processes all the way to manufacturing scale. The technology increases productivity as it can be run with smaller scale columns compared to the traditional batch chromatography for the same output. It is especially suitable for unstable molecules as the short process time and steady state operation support stability of the target product.
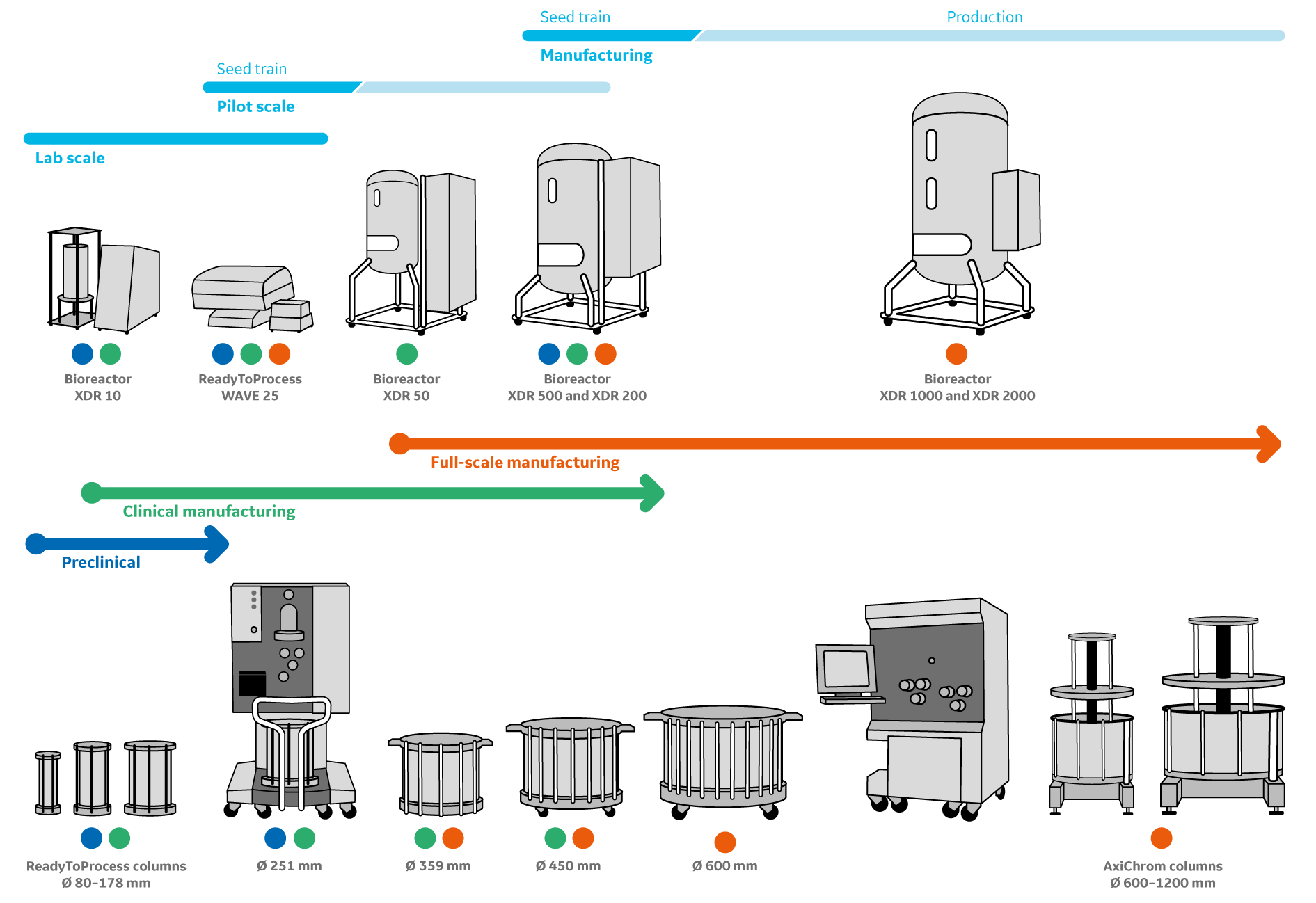
Figure 2: A scalable single-use chromatography platform: ÄKTA ready chromatography systems and ReadyToProcess columns can be used with Xcellerex XDR single-use bioreactors and XDUO single-use mixing systems. ÄKTA ready XL can also operate AxiChrom clean-and reuse columns with inner diameters of up to 1200 mm. Consistency in column geometries allows for convenient scaling, from early preclinical to commercial manufacturing scale.
For design of disposable purification processes from bioreactor harvests of up to 2000 L, ReadyToProcess columns are available with inner diameters of up to 450 mm. MabSelect PrismA protein A affinity resin offers several possibilities of operation for prepacked columns for mAb purification. Combined with the ReadyToProcess column format, the improved capacity of MabSelect PrismA can purify mAbs from bioreactor harvests of titers even higher than what was possible with its predecessor products.
Is It Ever Too Late To Make A Change?
Apprehension about making changes to a biopharmaceutical process is oftentimes related to concerns about regulatory acceptance. This is because stringent requirements from the FDA have historically made continuous improvements difficult. Companies are hesitant to revisit existing processes due to the fear they could experience costly delays or even rejection as a result. However, over the last decade, the agency has become more supportive of emerging technologies and has taken steps to drive innovation, such as the creation of its Emerging Technology Team. This group was organized “to promote the adoption of innovative approaches to pharmaceutical product design and manufacturing.”1 FDA Commissioner Scott Gottlieb and Director of CDER Janet Woodcock released a joint statement earlier this year confirming their stance on continuous manufacturing, which they say “helps to ensure consistently-made products, allows manufacturers to more easily scale their manufacturing operations to meet demand, and can help reduce drug shortages by minimizing operational stops and starts.”2
Nevertheless, the further you are in your manufacturing process, the more difficult it can become to make a change. It is truly dependent, though, on the magnitude of that change. For example, if you want to introduce a new chromatography resin in the late phases of your clinical trials, it will likely require considerable rework to your original process. But if it is only an equipment change, this should not require a major impact from a timeline or cost standpoint. The vendor you work with should be able to guide your decision-making process by first gaining a deep understanding of your process and how it can be optimized and then modifying the operating conditions to get the most out of the solution they are providing. If they cannot, you run the risk of designing your facility or manufacturing train in a way that does not meet the technical needs of the project, ultimately leading to product and even company failure.
When considering making changes to your biomanufacturing process, it is critical to complete an assessment of your in-house capabilities to determine if your company can implement the desired changes long term. Any gaps in technical capabilities or resources can lead to bottlenecks that may put your timeline in jeopardy. Having support from an equipment provider that offers not just innovations in biomanufacturing equipment but also an end-to-end biomanufacturing capabilities moves the relationship well beyond a transactional one; instead, it serves as an invaluable tool that can help prepare you for the independence needed to control your own manufacturing destiny. Therefore, making changes to your biomanufacturing process can be intimidating, but it is never too late as long as you have a trusted partner with the expertise and experience to guide you toward success.
- https://www.fda.gov/aboutfda/centersoffices/officeofmedicalproductsandtobacco/cder/ucm523228.htm +
- https://www.fda.gov/news-events/press-announcements/fda-statement-fdas-modern-approach-advanced-pharmaceutical-manufacturing
Discover more in the Process Efficiency series:
- Optimizing process efficiency in upstream manufacturing
- 4 Steps Toward End-To-End Connected Manufacturing
- Achieving operational efficiency in today’s fragmented market
- Intensified chromatography strategies
- Process Intensification