Eric Wiktelius, Applications specialist
Tangential flow filtration (TFF) is an important tool in the downstream processing of biopharmaceuticals. In the large-scale single-use product line for TFF, we have recently launched a TFF cassette filter holder, connecting tubing manifold, and sample distribution plate, which eliminates contact with multiuse surfaces before and after passing through the filter. The holder can be used with at least 10 m2 surface area membranes and matches the feed flow of the ÄKTA readyflux™ XL tangential flow filtration system. To demonstrate the combination of our single-use solutions for TFF, we developed a model concentration and diafiltration process of bovine γ-globulin using ÄKTA readyflux™ XL tangential flow filtration system with T-series Centrasette™ TFF cassettes. We used Omega™ polyethersulfone (PES) membranes for a 500 L initial sample volume. Using this approach, we achieved a 10-fold reduction in volume, followed by a four-fold buffer exchange in less than 2 h using 5 m2 of filter surface area.
Find out more about our solutions for tangential flow filtration using ÄKTA readyflux™ systemsIntroduction
TFF is a fast and efficient method to concentrate and purify your product utilizing ultrafiltration membranes. In addition, TFF can be used to buffer exchange your sample solution preparing it for the next purification step in your process. The process we describe can be modified to fit your processes and is a complete solution for concentration and diafiltration. We designed the setup as a disposable process, with the filtration system using a disposable flow path, including that of the filter holder. The buffer and sample were stored in single-use bags. We also chose to use TFF cassettes because of their high flux rate, which is well-suited to applications such as concentration of protein solution.
Detailed Materials and methods for the experiment described are found at the end of the document.
Results
Table 1 shows the volumetric outputs from the results of the filtration procedure. The permeate was not saved, so its volume is based on the total permeate output from the flow meter. This information was also used to calculate the total amount of sample processed during fed-batch concentration. The volume of the recirculation path was kept the same throughout both concentration and diafiltration.
Table 1. Fraction and volumetric output during the filtration process
| Fraction | Volume (L) |
|---|---|
| Average retentate volume | 53.4 |
| Total permeate flow during concentration | 471.6 |
| Total sample volume loaded during fed-batch | 525 |
| Concentration factor | 9.8 |
| Total permeate flow during diafiltration | 214.0 |
| Diafiltration factor | 4.0 |
Table 2 shows the absorbance at 280 nm (A280) measurements performed which proportionally corresponds to the protein concentration. Since the permeate volume was not saved, no A280 data is available, but the UV sensor in the permeate flow path detected near zero absorbance.
Table 2. A280 measurements for the different fractions during the TFF process
| Fraction | Volume (L) | A280 | Yield (%) |
|---|---|---|---|
| Start sample | 525 | 1.09 | 100 |
| Concentrated and diafiltered sample | 53.4 | 10.36 | 96.4 |
| Wash sample | 10 | 2.60 | 4.5 |
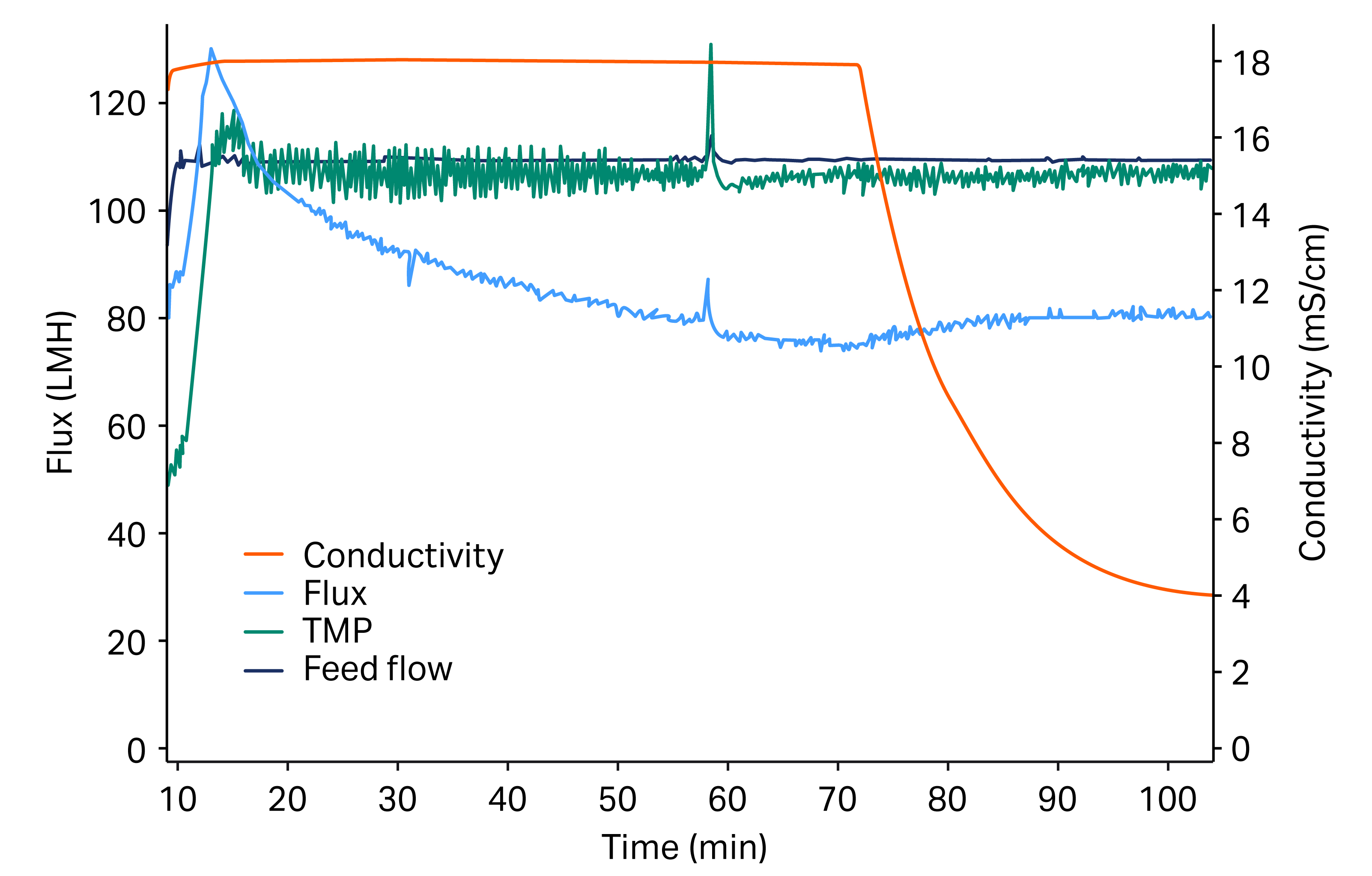
Fig 1. Graph of concentration and diafiltration during the TFF process. Flux is shown in light blue in LMH (L/m2/h). The feed flow (dark blue) was fixed at 30 L/min and transmembrane pressure (TMP) control (green) remained stable at 1 bar (14,5 psi, 0.1 MPa) throughout.
Figure 1 shows the concentration and diafiltration phases of the TFF process. The initial transfer of sample, recirculation, and drain were omitted. The permeate flux dropped after stabilization of TMP from around 110 LMH to 75 LMH during concentration. At the start of the diafiltration, the flux initially increased and then stabilized. The sharp peaks observed in the middle of the process were due to a process-unrelated artifact. At the start of the diafiltration, the conductivity in the retentate dropped and then leveled out towards the end as NaCl was being washed out. The whole process was finished in approximately 2 h.
Conclusions
We have shown a successful concentration and diafiltration process using ÄKTA readyflux™ XL filtration system with T-series Centrasette™ TFF cassettes with Omega™ PES membrane for a 500 L initial sample volume.
- The filtration application was designed as a single-use process, using Xcellerex™ XDM mixer bags, Xcellerex™ XDUO 100 L mixing system, and ÄKTA readyflux™ XL filtration system. The single-use flow path of the filtration system was connected through the manifold to the distribution plate of the cassette holder, where the product passes the cassettes without contact with the holder endplates.
- The concentration and diafiltration of bovine γ-globulin were successful, reducing the volume from above 500 L to 50 L (10-fold), and changing four buffer volumes via continuous diafiltration in less than 2 h.
- The protein yield was nearly 100% as measured by A280 from samples before and after processing.
CY38038-080224-AN
Buffers and solutions
The diafiltration buffer and sample solution were prepared in a large 500 L stirred vessel and transferred into bags afterward. Citric acid monohydrate (2.63 kg) and 4.69 kg glycine were weighed out and dissolved in distilled water to a total weight of approximately 220 kg. The pH was regulated with the addition of NaOH solution until pH 4.0 was reached and the total weight was corrected to 250 kg with water. The buffer was transferred to a 200 L bin bag.
The equilibration buffer was prepared in a 50 L polypropylene barrel with an immersed stirrer. Citric acid monohydrate (525 g), 938 g glycine, and 438 g NaCl were weighed into a 50 L barrel with an immersed stirrer, and approximately 40 L of distilled water was added. The pH was regulated with the addition of NaOH solution until pH 4.0 was reached and the total weight was corrected to 50 kg with water.
Sample protein solution
Bovine γ-globulin (550 g), 5.78 kg citric acid monohydrate, 10.32 kg glycine, and 4.82 kg NaCl were weighed out and mixed with distilled water to a total weight of 520 kg. The solution was mixed thoroughly to dissolve the protein fully and the pH was set to 4.0 with NaOH, diluted as much as possible to not overshoot the final sample volume. When the solution was deemed homogenous and the pH was regulated, the solution was passed through ULTA™ Pure SG 30 in. 0.2 µm filter (KMP-SG9230TT), using a peristaltic pump and silicone tubing, and directed into the 500 L bin bag.
Methods
We designed the filtration system as a single-use process using Xcellerex™ XDM mixer bags, Xcellerex™ XDUO 100 L mixing system, and ÄKTA readyflux™ XL filtration system (Fig 2). A single-use flow path was used to connect the manifold to the distribution plate of the cassette holder, and where the product passed through the cassettes it did not have contact with the endplates of the holder.

Fig 2. Overview of the single-use process using Xcellerex™ XDM mixer bags, Xcellerex™ XDUO 100 L mixing system, and ÄKTA readyflux™ XL filtration system.
The 100 L bag used for recirculation was a custom bag for the Xcellerex™ XDUO 100 L single-use mixing system with an added retentate return on top of the bag. This return was not used. Instead, one of the ¾ in. probe ports was removed from the barbed fitting and was used to mount a 1 in. reinforced silicone tubing long enough to reach the retentate return on the flow kit. This created a submerged return of the retentate to avoid foaming.
All methods excluding the wash were written as UNICORN™ software method sequences. The hold-up volume in the flow path including filters was determined to be 3.4 L from earlier experiments by measuring the volume that decreased from the recirculation chamber as a result of filling the retentate pathway.
Flat sheet cassette and flow path installationThe cassettes were installed in the holder on each side of the distribution plate, compressed using the automatic torque functionality of the holder, and connected with the connection manifold to the ÄKTA readyflux™ XL filtration system flow kit (Fig 3). The recirculation tank was connected to the Feed Tank inlet, water to Feed 1, and equilibration buffer to Feed 2. Sample added during the fed-batch operation was connected to Transfer 1 and the diafiltration buffer to Transfer 2.
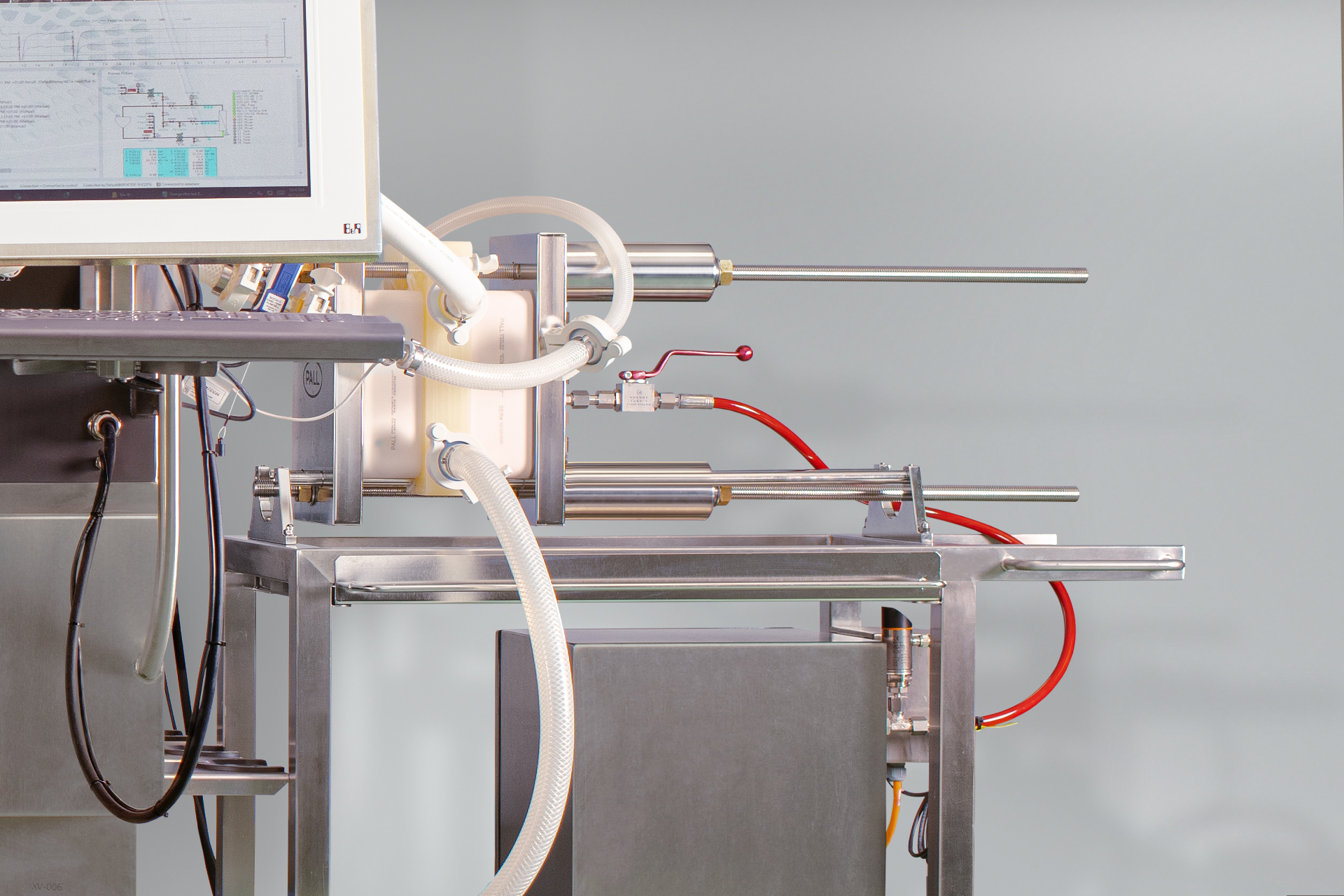
Fig 3. T-series Centrasette™ TFF cassettes in a holder connected to ÄKTA readyflux™ XL filtration system.
FlushThe filters were flushed with distilled water at 30% manual feed flow with permeate and retentate fully open, and both directed to waste until a total of 50 L of feed flow had been reached. The system was drained before conducting the integrity test.
Integrity testThe Installation Switch was set to Integrity Test and the inlet for integrity testing was pressurized at 2 bar, (29.0 psi, 0.2 MPa). A bucket and a 2 L measuring cylinder were filled with water. The cylinder was quickly inverted, and the opening was placed under the surface of the water in the bucket. After the system had stabilized, the permeate outlet was directed to the submerged opening of the cylinder. The volume of air traveling through the permeate was measured by studying the graduation on the cylinder before and after 1 min. A total of 1500 mL air was collected, which meant the integrity test passed.
EquilibrationEquilibration of the filters was performed in the same way as the flush, but with buffer, and with a UV zeroing command after 1 min of processing. A normalized water permeability (NWP) calculation was also started at the same time and averaged 380 LMH/bar during the final stages of equilibration. The system was once again drained.
Sample filtration processThe aim was a 10-fold concentration at 30 L/min (360 LMH load) constant feed flow with a TMP of 1 bar, 14.5 psi, 0.1 MPa) followed by a four-fold diafiltration at the same filtration parameters. Since the recirculation tank had a 100 L maximum volume, the concentration was run as a fed batch keeping the volume in the feed tank at around 50 L. The process involved the following steps:
- The Xcellerex™ XDUO 100 L single-use mixing system weight was tared when the bag was installed.
- The sample connected to Transfer 1 was loaded via the transfer pump at 50% manual flow into the Xcellerex™ XDUO 100 L single-use mixing system until the retentate volume was 60 L.
- The agitator of the XDUO mixing system was started with 50% clockwise stirring.
- The sample was recirculated with permeate closed for 3 min at 25% manual feed flow with the retentate pressure control valve (PCV) fully open.
- The permeate line was opened, feed flow was set to 30 L/min and TMP was set to of 0.1 MPa (1 bar, 14.5 psi) and the UNICORN™ software monitored the concentration factor. The feed tank level maintenance setpoint was set to 50 L.
- The concentration phase was manually interrupted at around a concentration factor of 9.8 and the diafiltration commenced under the same running parameters.
- The software monitored the calculated diafiltration factor until it reached 4 and then proceeded to recirculation only mode with closed permeate and completely open retentate PCV at 30 L/min.
- After 3 min of recirculation the system was drained, first with feed pump action to drain the recirculation chamber, and then with air assisted draining of the retentate pathway using the transfer pump.
A 10 L volume of diafiltration buffer was added to the recirculation tank directly after emptying and recirculated for 5 min with the permeate closed. A manual feed flow was maintained between 10% and 20% to avoid foam formation, but provide adequate mixing. No agitator was used because of the low volume.