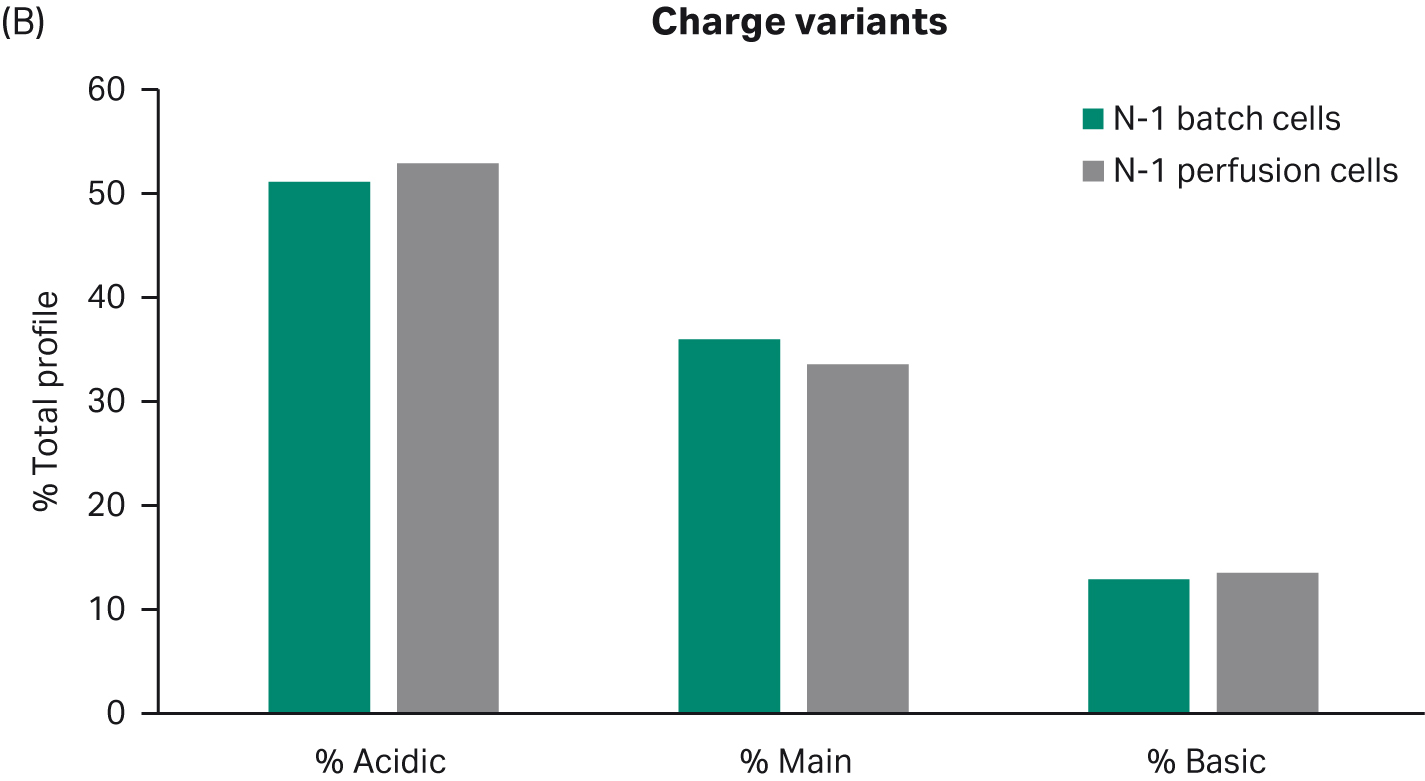A high-density N-1 seed culture can improve your fed-batch process by allowing you to seed the production (N) bioreactor at a higher starting density or to replace a large N-1 bioreactor. This study shows how to prepare such a culture using the Xcellerex Automated Perfusion System (APS) along with a 50 L capacity Xcellerex stirred-tank bioreactor. The process we developed generated a final cell density of 179 million viable cells (MVC)/mL. The cells cultured by perfusion, as well as cells cultures by standard batch process in N-1, were then used to seed fed-batch cultures. The results show that introducing perfusion in the N-1 step does not affect growth, productivity, or product quality profile.
Introduction to N-1 perfusion
N-1 perfusion refers to the intensification of the final seed train step by introducing perfusion. A perfusion process supplies the cell culture with a continuous flow of fresh media and removes spent media while retaining the cells in the bioreactor.
A high-density N-1 seed culture process can be used to improve a fed-batch process by seeding the production bioreactor at a higher starting density (1‒3). Or it can replace a larger N-1 bioreactor to seed one or more production bioreactors. As a result, you can increase a facility’s production rate, decrease investment costs, and reduce facility footprint, all of which reduce production costs.
In this study, we thawed frozen mAb-producing CHO cells and subcultured them in shake flasks. Next, we transferred cells to a ReadyToProcess WAVE 25 bioreactor for the N-2 step. Then, we used this culture to seed an XDR-50 bioreactor for an N-1 perfusion step using the Xcellerex APS. Finally, we seeded fed-batch cultures in shake flasks using the perfused cells. We performed the fed-batch step as a proof of concept that cells retain their capacity to grow and produce after the intensified perfusion step in N-1.
Results and discussion
N-1 perfusion in Xcellerex XDR-50 using the APS skid
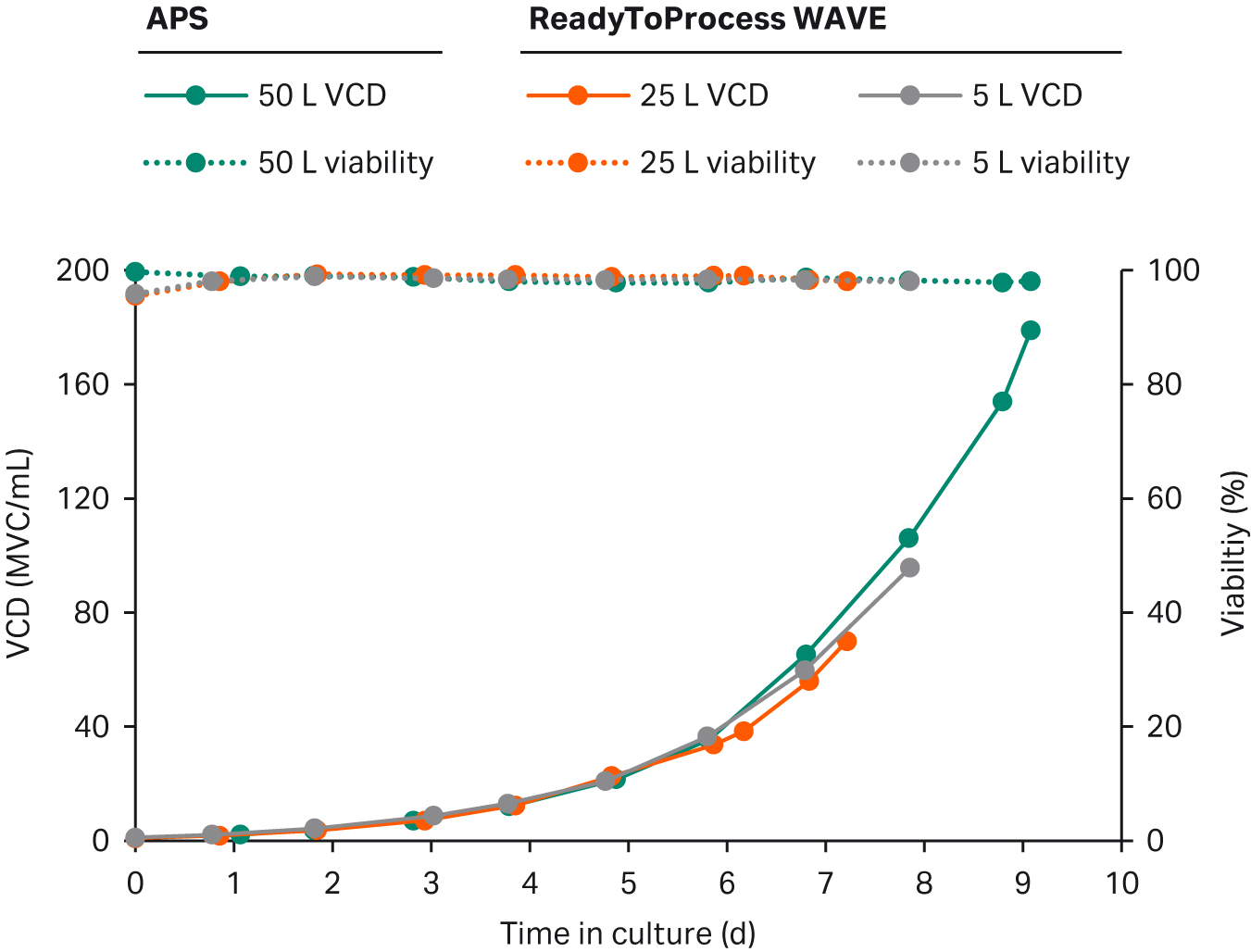
We previously developed an N-1 perfusion process at 5 L and 25 L scales in a ReadyToProcess WAVE 25 bioreactor. At both scales a final density in the range of 70 to 95 MVC/mL was achieved with exponential growth and stable DO control throughout. Here, we scaled the process to the XDR-50 bioreactor.
Cells grew exponentially throughout the run to a cell density of 179 MVC/mL in 9 days.
Scalability of the perfusion process ‒ ReadyToProcess WAVE 25 to XDR-50
To evaluate the scalability between these two types of bioreactors, we collated all the growth data — from previous 5 L and 25 L perfusion culture in ReadyToProcess WAVE 25 and from the developed perfusion culture in XDR-50 (Fig 1).
The data sets show nearly perfect alignment for all three cultures. In the WAVE bioreactor, DO regulation was challenged as cell densities exceed 95 MVC/mL. The DO regulation did not reach its maximum capacity during the XDR-50 run (data not shown). Thus, although the scalability from rocking to stirred-tank bioreactor is good, the maximum cell density that can be reached is most likely higher in the XDR-50 resulting from the more efficient oxygen transfer.
Fig 1. Growth and viability for the intensified N-1 perfusion process in ReadyToProcess WAVE at 5 L and 25 L working volumes (wv), and at 47 L wv in XDR-50.
Fed-batch process with perfused vs batch-cultured cells
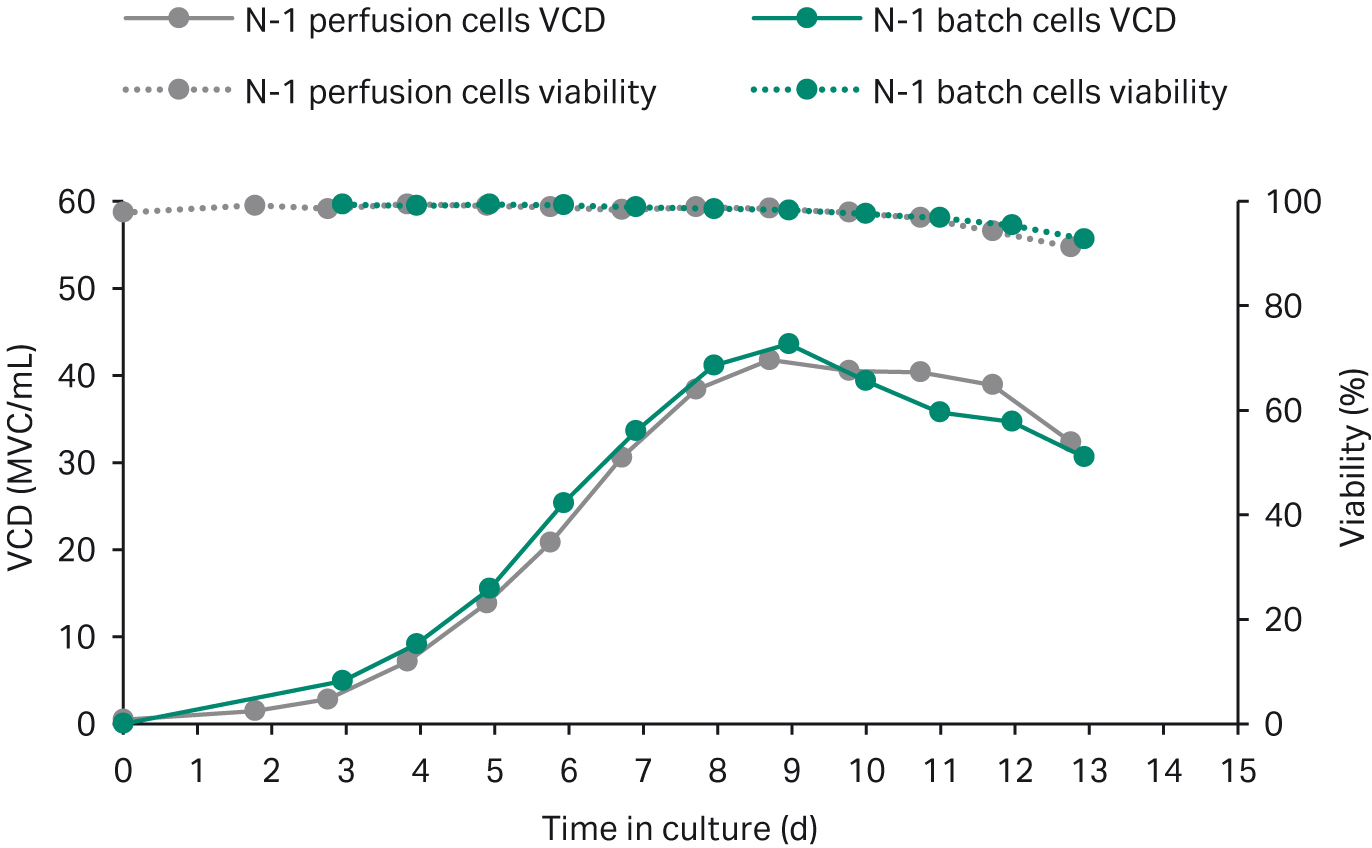
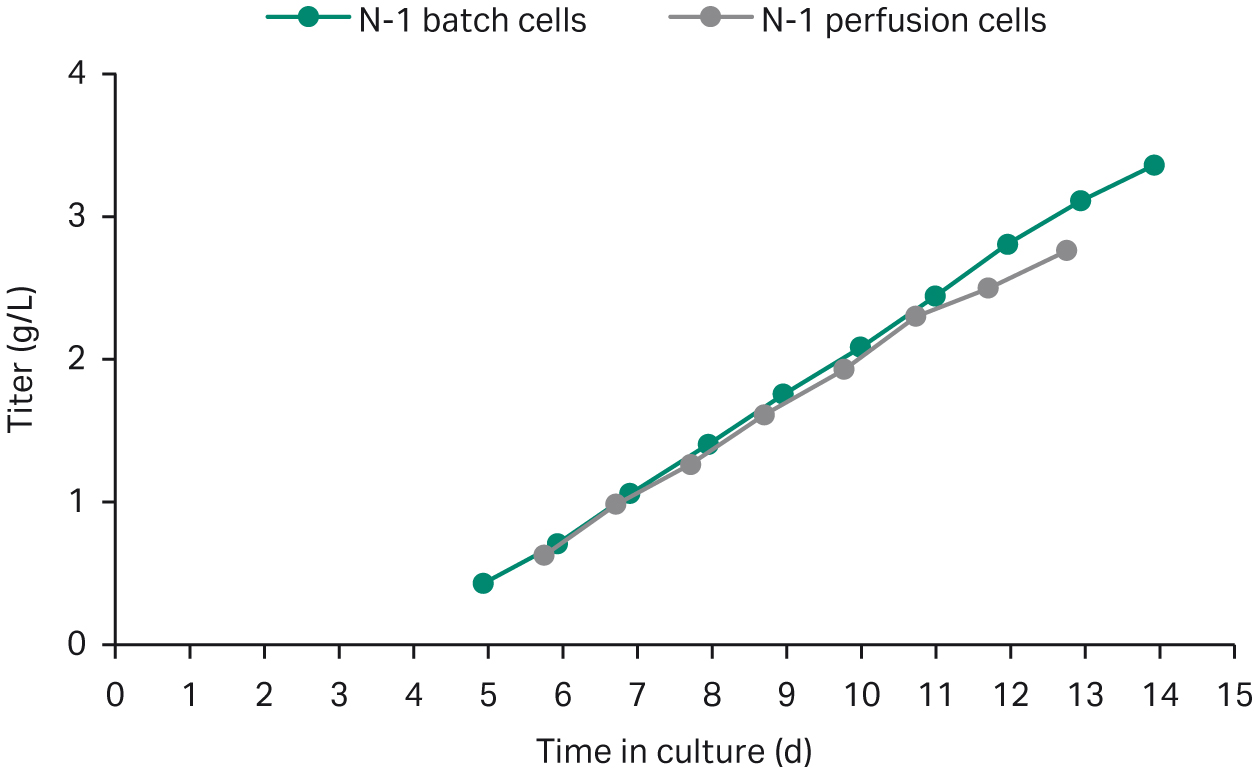
We harvested a small fraction of the perfusion culture at 179 MVC/mL and used this to seed shake flasks cultured in fed-batch. For comparison, we seeded two shake-flasks using batch-cultured N-1 cells and cultured them using the same fed-batch process. The growth and productivity data from both batch and perfusion seeded fed-batch processes are shown in Figures 2 and 3, respectively. Growth, viability, and titers align well between perfused and batch-cultured cells, with a minor difference in titer that is within the normal batch-to-batch variation.
Fig 2. Growth and viability data for fed-batch cultures in shake flasks seeded with perfused cells and batch-cultured cells.
Fig 3. Titer data for fed-batch cultures in shake flasks seeded with perfused cells and batch-cultured cells.
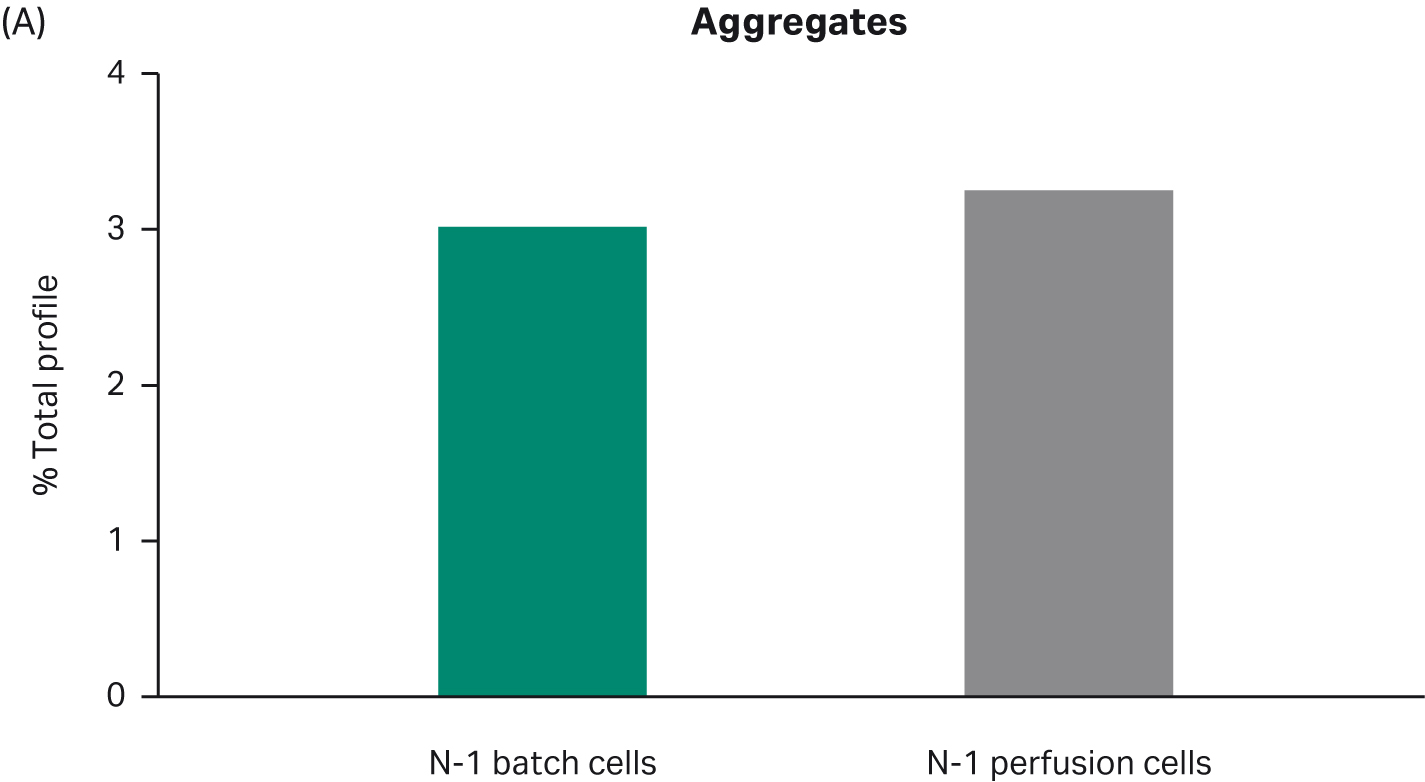
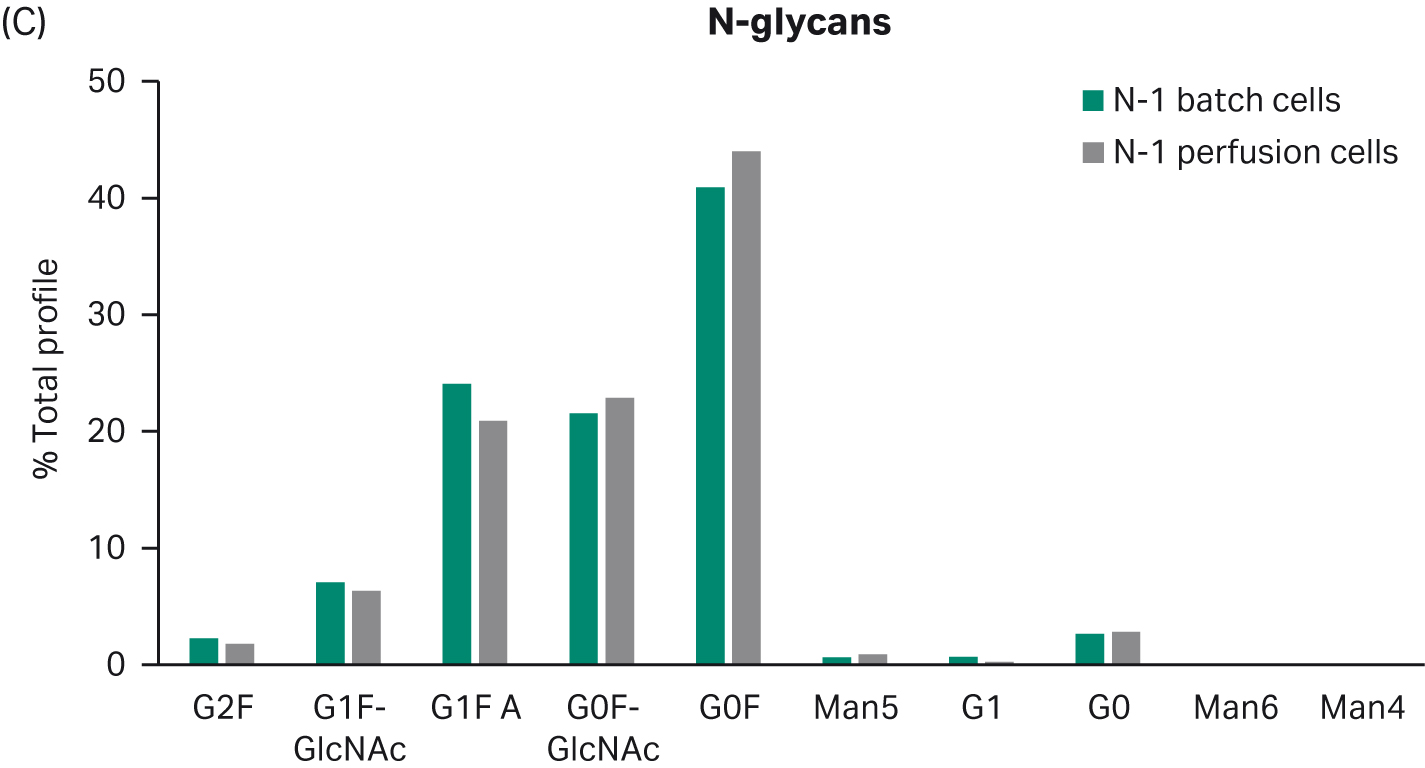
Further, harvested samples analyzed for product quality profiles show comparable levels of aggregates and charge distribution, as well as comparable N-glycan profiles (Fig 4).
(A)
(B)
(C)
Fig 4. Product quality profiles for fed-batch cultures in shake flasks seeded with perfused cells and batch-cultured cells. (A) aggregates, (B) charge distribution, and (C) N-glycan profile.
There are many benefits of introducing N-1 perfusion to intensify the seed train. For example, the high cell-density culture can be used to seed production processes at higher cell densities. A higher seeding density pushes the cell accumulation to the less expensive seed reactor and shortens the production process, which increases the production capacity. Perfusion can also allow use of a smaller N-1 bioreactor, saving expenses as well as floor space. Of course, a perfusion process also increases the volume of cell culture media consumed and adds complexity to the process, both of which must be considered when designing the upstream process.
Despite the benefits of N-1 perfusion, this is only an option if it allows cells to maintain their capacity to grow and produce in the production step. Here, we show that the N-1 perfusion does not affect growth, productivity, or product quality in a fed-batch process. These results support use of the intensified seed train strategy to increase flexibility and productivity of the overall process.
We developed the perfusion process at two scales in the ReadyToProcess WAVE 25 system. Here we show that the viability and VCD of the perfused cells from these scales are comparable with those from an XDR-50 stirred-tank bioreactor operated in perfusion mode with the Xcellerex APS. Not only do these results demonstrate good scalability to larger volumes, but they also show the potential for ReadyToProcess WAVE 25 as a scale-down model for the Xcellerex APS.
References
- Brunner M, Fricke J, Kroll P, Herwig C. Bioprocess Biosys Eng. Investigation of the interactions of critical scale-up parameters (pH, pO2 and pCO2) on CHO batch performance and critical quality attributes. 2017 40(2):251-263. doi: 10.1007/s00449-016-1693-7.
- Yang W, Lu J, Kwiatkowski C, et al. Perfusion seed cultures improve biopharmaceutical fed-batch production capacity and product quality. Biotechnol Prog. 2014 30(3):615-625. doi: 10.1002/btpr.1884.
- Padawer I, Ling W, Bai Y, et al. Case study: An accelerated 8-day monoclonal antibody production process based on high density seeding densities. Biotechnol Prog. 2013 29(3):829-832. doi: 10.1002/btpr.1719.
Materials and methods
A Chinese hamster ovary (CHO) cell line expressing the immunotherapeutic mAb, Herceptin®, was used in all experiments. ActiPro medium (Cytiva) with 37.5 μM methionine sulfoximine (MSX) was used in all seed train steps in shake flasks. MSX was not added to seed train steps run in bioreactor scale.
Seed train in shake flasks
Cells were thawed and passaged in shake flasks every 3 to 4 d. The final density at each passage was not allowed to exceed 7 MVC/mL to ensure constant exponential growth. For the same reason, cells were kept at a seed density above 0.5 MVC/mL.
Seed train in ReadyToProcess WAVE 25 bioreactor
The N-2 culture was performed in batch in a ReadyToProcess WAVE 25 bioreactor equipped with a 20 L cell culture bag (Cytiva). The reactor was seeded at a density of 0.5 MVC/mL in a total volume of 10 L and cultured for 3 d, controlled at 40% DO and a temperature of 37°C. Target criteria were 5 × 106 cells/mL at a viability of > 95% and a population doubling time of < 40="" />
N-1 perfusion in XDR-50 bioreactor
The N-1 perfusion was performed using an XDR-50 bioreactor with a development bag with 2 µm, 20 µm, 0.5 mm, and 1 mm drilled hole spargers (Cytiva). The 20 µm sparger was used for oxygen supply, and the 1 mm sparger was used to allow for the efficient stripping that is required to achieve high cell densities. The bioreactor was seeded with cells from the N-2 ReadyToProcess WAVE 25 culture at a final volume of 47 L at a cell density of 1.0 MVC/mL.
Cells were expanded for 1 d. Thereafter, at a cell density of 2.0 × 106 cells/mL, perfusion was initiated and maintained at a cell-specific productivity rate (CSPR) of 40 pL/cell/day using a media mixture consisting of ActiPro medium, Cell Boost 1 and Cell Boost 3 supplements, and 2 g/L poloxamer. The culture was controlled at a pH of 6.8, 40% DO, and a temperature of 37°C. Target criteria were 100‒200 × 106 cells/mL at a viability of > 95% and a population doubling time of < 40="" />
The process in ReadyTo Process WAVE was developed using a strategy where the perfusion rate was set once per day at a CSPR based on the estimated VCD in 12 h. The XDR-50 software Wonderware™ offers the opportunity to program the rate of the media pump via the set point table. For this run, we used this opportunity to further trim the perfusion rate to follow the cell growth by calculating the perfusion rate based on the estimated VCD in 12 h in 2 h increments. This way, the perfusion rate plotted over time results in an exponential increase that mimics the growth curve of the culture.
The media exchange rate was set using the set point table for the media pump. After daily sampling, the VCD was used to calculate the media addition rate for maintaining a CSPR of 40 pL/cell/day. The rate was calculated in 2 h increments based on the estimated VCD in 12 h to allow for adjustments in the flow rate to mimic the exponential growth rate of the cell.
Fed-batch cultures in shake flasks
Exponentially growing cells were harvested at 179 MVC/mL for fed-batch cultures in shake flasks. Cells were seeded in ActiPro medium at a cell density of 0.5 MVC/mL. In parallel, shake flasks were seeded with cells from a batch culture at the same density as a baseline. Feeding was started on day 3. The daily feed volumes of Cell Boost 7a and Cell Boost 7b, relative to the initial working volume, were 2.5% and 0.25% respectively. Glucose was added to a final concentration of 4 g/L when levels decreased below 2 g/L at sampling. Target criteria were 40–50 MVC/mL at a viability of > 90%.
Analysis
Samples were taken from the reactors and analyzed daily. VCD and viability were measured using Vi-CELL™ XR (Beckman Coulter). pH, pO2, pCO2, and osmolality were measured using an ABL9 blood gas analyzer (Radiometer). Product titer, nutrients, and metabolites were all measured on a Cedex™ Bio Analyzer (Roche).
Protein quality analysis
Samples from the fed-batch cultures were analyzed for product quality and documented according to Table 1.
Table 1. Product quality analysis and the internal methods used
| Parameter | Analysis method |
| Charge variants | Cation exchange chromatography |
| Aggregates | Size exclusion chromatography |
| N-glycans | Multi-attribute method-mass spectrometry (MAM-MS) |
TR#29663423