Principles and methodologies for biopharmaceutical development and manufacturing are well established today. However, the increased molecular diversity and drive for higher productivity brings new challenges for process developers. One of these challenges is how to get deeper understanding for sources of variability and set mitigation strategies.
This article shares some of our insights about how raw material variability is a potential source for process variation. The focus lies on chromatography resins.
Drivers for increased process understanding
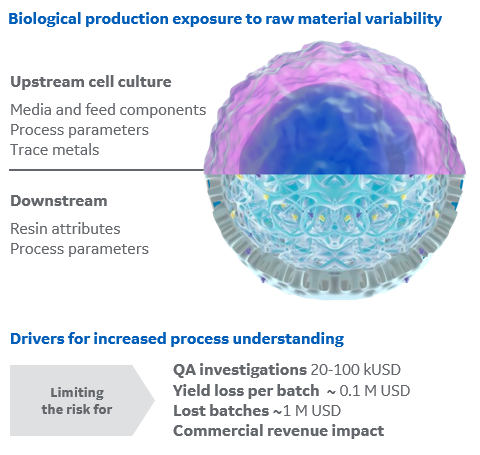
Cell culture media components and resin attributes can have significant impact on both process economics as well as product quality.
With a deeper process understanding and raw material insights, you can minimize the risk of yield losses, lengthy batch investigation and troubleshooting, or even batch rejection and hence high revenue losses. Small investments in process development can help maximize future process robustness (Fig 1).
Fig 1. Overview of some of the drivers for increased process understanding and sources for process variation.
Chromatography resin variability: an industry blind spot?
Over the past decade, using a quality by design (QbD) approach to biopharmaceutical process development has helped ensure patient safety and drug efficacy. QbD incorporates the identification of critical quality attributes (CQAs) and links to critical process parameters (CPPs) and critical material attributes (CMAs).
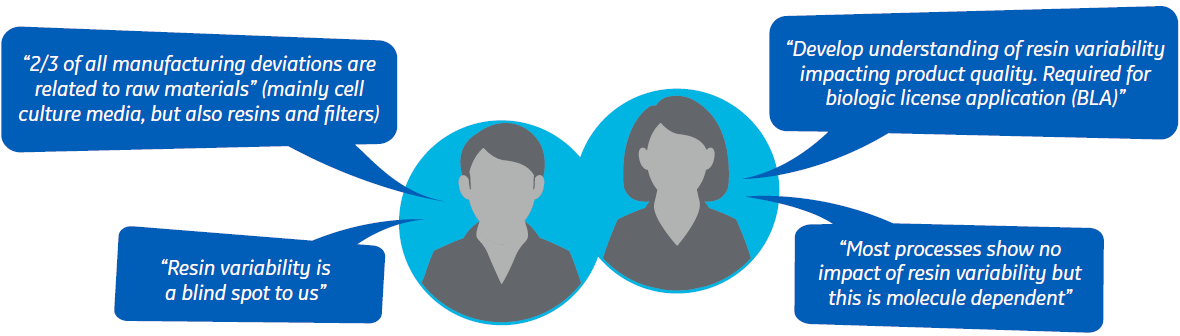
Chromatography resin properties are sometimes suggested as critical raw material attributes that potentially could impact CQAs (Fig 1). They are also considered important parameters for consistent downstream chromatography process performance. However, the effect of resin variability on process robustness is still an industry blind spot.
Fig 2. What some process developers say about the interplay of process parameters with resin variability.
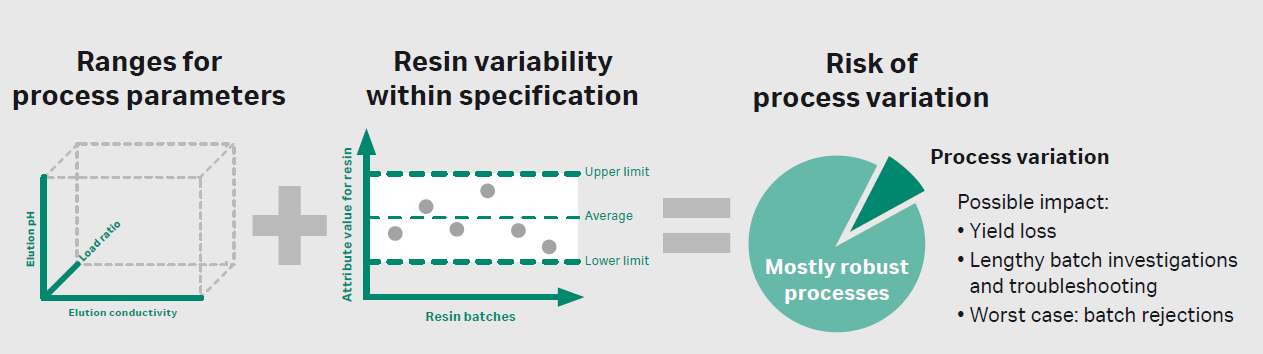
Chromatography resins are always produced against a specification interval. It is in the hands of the process developers to investigate whether the inherent variability, within a manufacturer’s given specification range, has an impact on process outcomes such as yield or productivity (Fig 3). There are several examples published in the literature.
Fig 3. Illustration of the interplay between process parameters and resin attributes and its impact on process robustness.
A growing focus on process understanding
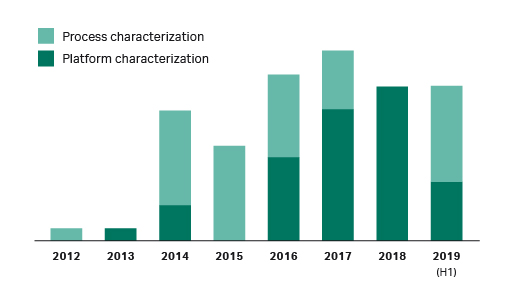
As a resin supplier, we have seen an increase in process robustness collaborations with biopharmaceutical companies (Fig 4). Typically, these collaborations are driven by a need to understand the potential impact of resin variability on CQAs or downstream process performance indicators.
Furthermore, during interviews with process developers in 2018, we found that seven out of nine had seen impact of resin properties on purification performance. Together, this indicates that there is an increased focus on process understanding in the industry with regards to chromatography resins variability.
Fig 4. Trends in process robustness collaborations between Cytiva and biopharmaceutical companies.
Challenging separations are more impacted by resin variability
Including resin variability as part of process characterization helps to minimize the potential impact on purification performance. However, these studies are not required for all processes. A risk-based approach is suitable to guide when to include resin variability as part of characterization studies.
Resin ligand density is regarded as the resin attribute most likely to impact process performance. This is especially true for challenging purifications. Resin variability has been found more likely to impact:
- processes using hydrophobic interaction chromatography (HIC) and multimodal chromatography (MMC) techniques compared to ion exchange chromatography (IEX)
- processes using bind/elute mode compared to flow-through mode
How to approach the challenge of resin variability
Resin variability needs to be viewed in the context of any other parameters that could influence quality attributes or process performance. Although process parameters typically have a higher risk of impact than resin attributes, understanding the interplay between the two is the key to maximizing robustness at manufacturing scale.
There are several topics to consider when approaching chromatography process development and characterization, such as:
- Use and extend platform knowledge—an efficient way to reduce risk while still facilitating speed
- Adopt a risk-based approach—to help identify when it is beneficial to include resin variability in process characterization
- Stay close with your resin supplier—as outlined in the BPOG white paper (1)
For more insights into process characterization, download this white paper on how to ensure process robustness in chromatography.
Related content
- White paper: Quality by design in biotherapeutics purification: understanding and addressing sources of process variability
- Risk assessment: studying chromatography resin variability
- 5 control strategy options to prevent resin variability impact
- Infographic: How to ensure process robustness in chromatography
References
- BPOG white paper: Patient-centric requirements for the supply of raw materials into biopharmaceutical manufacturing, BPOG, (2017)