What is polishing chromatography?
Polishing chromatography is the term we use to describe the removal of minute amounts of impurities in the final phase of biopharmaceutical manufacturing. This article shares the factors you should consider when developing your polishing steps — from the basics to the typical challenges faced.
When is polishing chromatography used?
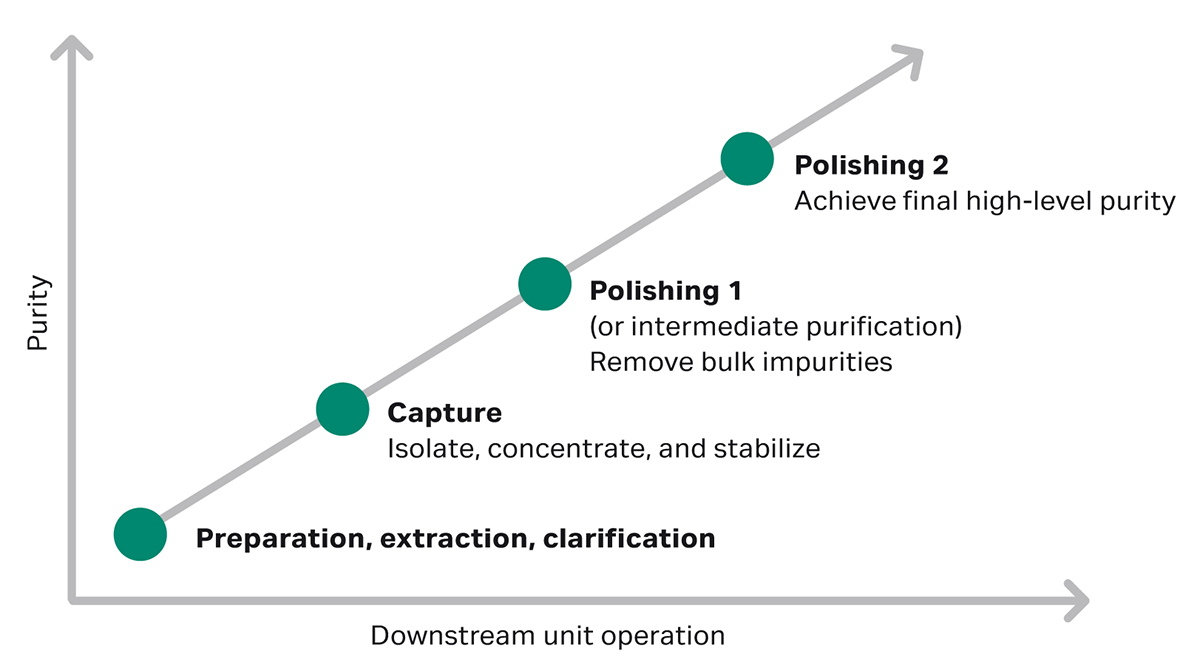
In the downstream bioprocessing phase of biopharmaceutical manufacturing, the first chromatographic capture step isolates the product from the bulk of impurities, such as cell culture media components and proteases. The next step is polishing chromatography, which will help you obtain higher purity of the target molecule by clearing remaining impurities (Fig 1).
The most efficient way to reduce impurities is to start purification with an affinity step as first capture step, whenever possible. In monoclonal antibody production, protein A affinity chromatography is widely used for the capture step. Purity above 95% may be achieved after this first step, which means that only limited polishing is required before the final formulation steps. However, most processes involving antibody-related products (mAbs, bispecific antibodies, Fab fragments) and other recombinant proteins require at least two polishing steps after the capture step.
For some other types of molecules affinity chromatography solutions may not always be available. Nowadays, purification of tagged proteins is mostly not considered for biomanufacturing because there is currently no satisfactory solution to remove all traces of the tag after the purification.
If no affinity chromatography solution is available for the target molecule, the second purification step should be designed to remove the bulk of process- and product-related impurities such as high molecular weight impurities (HMW), host cell proteins (HCPs), and DNA. If the capture step isn’t based on affinity chromatography, the step between capture and the final polishing step can also be called “intermediate purification” (Fig 1).
Fig 1. Strategy for protein purification.
In research, one polishing step might give you the purity you need. In clinical applications on the other hand, stringent regulatory approval means that you need to reduce impurities to trace levels. Furthermore, for viral clearance, a certain log-reduction of viruses over the complete process must be demonstrated. Combining multiple chromatography steps with orthogonal separation techniques contributes to virus reduction.
What are the typical impurities removed during polishing steps?
- Product-related impurities
- Host cell proteins (HCP)
- DNA
- Protein A leakage from previous capture step
- Viruses (viral clearance)
- Endotoxins (if using bacterial expression systems)
Typical techniques used for polishing
The typical chromatography techniques used for polishing are:
- ion exchange chromatography (IEX)
- hydrophobic interaction chromatography (HIC)
- multimodal (or mixed mode) chromatography (MM)
Ion exchange chromatography: the preferred starting point
IEX is usually the preferred starting point for polishing because it requires less optimization work. IEX is also perceived as more predictable than other polishing techniques, because of the apparent simplicity of the electrostatic interactions compared to the hydrophobic forces and mixed mode interactions involved. In reality, the use of modern screening tools and/or DoE methodology make MM and HIC very approachable.
For mAb polishing, cation exchange chromatography (CIEX) is commonly used in bind-and-elute (B/E) mode for the target molecule, which removes negatively charged impurities such as residual HCPs during sample loading or in the wash fraction. CIEX also has the power to separate antibody charge variants, leached protein A, and HMW molecules such as aggregates during elution.
Most mAbs are relatively basic molecules that can be polished using anion exchange chromatography (AIEX) in flow-through (FT) mode. The conditions used in FT mode promote binding of viruses, DNA, and acidic HCP to the resin ligand while the target molecule is washed through in the chromatography flowthrough and collected (1).
Hydrophobic interaction and multimodal chromatography
HIC is often used in intermediate and polishing steps where its selectivity can be fully utilized. For mAb purification, HIC is often used in FT mode This works especially well to remove large molecules such as aggregates.
For target molecules where IEX (preferably CIEX) is used for capture (when no affinity capture step is available), HIC and/or MM are commonly chosen as intermediate and polishing steps to ensure orthogonal combination of techniques.
Mixed mode chromatography has found a role in many processes replacing traditional chromatography steps. Mixed mode resins are most often used where the single-interaction resins have either failed to deliver desired results or have not done so at a reasonable cost (1).
Multimodal resins come in major variants: MM AIEX, and MM CIEX. They both have the benefit of being able to bind at higher conductivities than a regular ion exchanger. Furthermore, MM AIEX resins sometimes also bind below the isoelectric point (pI) of the drug substance. Thus, MM resins are preferred when:
- the loading conductivity of the sample is too high for a traditional ion exchange resin
- when the number of purification steps needs to be kept a minimum
- a different selectivity is needed than that provided by the individual ionic or hydrophobic interaction types alone.
Even if the development of MM or HIC steps is less straightforward than with IEX, the right conditions can be identified with a few high-throughput experiments, see the next section for details.
Platform approach: a way to standardize process development
A platform approach makes process development faster and more predictable. Platforms typically include certain standard operating procedures (SOPs), checklists, equipment, chromatography techniques and materials to be used including the chromatography resins. If you have several molecules to purify, reusing a similar protocol for all will save time. This is a great way to standardize drug development and manufacturing and make development of polishing steps straightforward.
When it comes to chromatography resins, always have a backup palette of alternative chromatography resins to evaluate if the purification becomes more challenging than you thought. Note that when using an existing platform for a new target of the same class of molecules, the platform approach is a good starting point to obtain the desired purity of material for further studies. However, the process may not be optimized with respect to yield, which is sub-optimal from a long-term economic standpoint.
In the end, all critical quality attributes must meet their specified levels. No compromise on purity/safety and efficacy can be allowed, but the efforts spent to optimize yield for long-term economy must be balanced with time-to-market requirements.
Each process must be thoroughly characterized and the relations between process parameters and critical quality attributes of the product need to be well understood. For the design of a non-platform process, the best place to start is with careful characterization of the target product and its impurity profile, followed by a risk assessment that leads to prioritization of the purification process tasks.
For optimization aiming at securing high purity and high yield, the use of high-throughput techniques is well established. Parallel and miniaturized techniques are beneficial because they require relatively low amounts of test material while generating large amounts of information. These methods are often used together with statistical models that increase process understanding but do not fully explain the results. For deeper understanding, advanced methods such as mechanistic modeling are emerging.
mAb purification platforms
The goals of polishing in mAb purification are to:
- Remove mAb aggregates
- Remove unwanted mAb charge variants or other product related impurities
- Remove HCP
- Remove leached protein A from the capture step
- Remove host cell DNA
- Contribute to viral clearance
Typical mAb purification
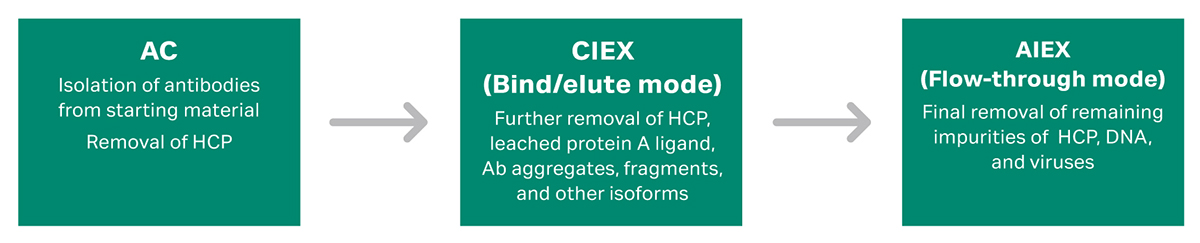
A typical mAb purification platform will start with an affinity capture step, followed by two polishing steps. These polishing steps are commonly CIEX and AIEX (Fig 2).
Fig 2. Example of a chromatography resin platform for monoclonal antibody (mAb) that involves one affinity capture step (AC) and two polishing steps (CIEX and AIEX). AC = affinity chromatography. CIEX = cation exchange chromatography. AIEX = anion exchange chromatography.
Alternative mAb purification processes
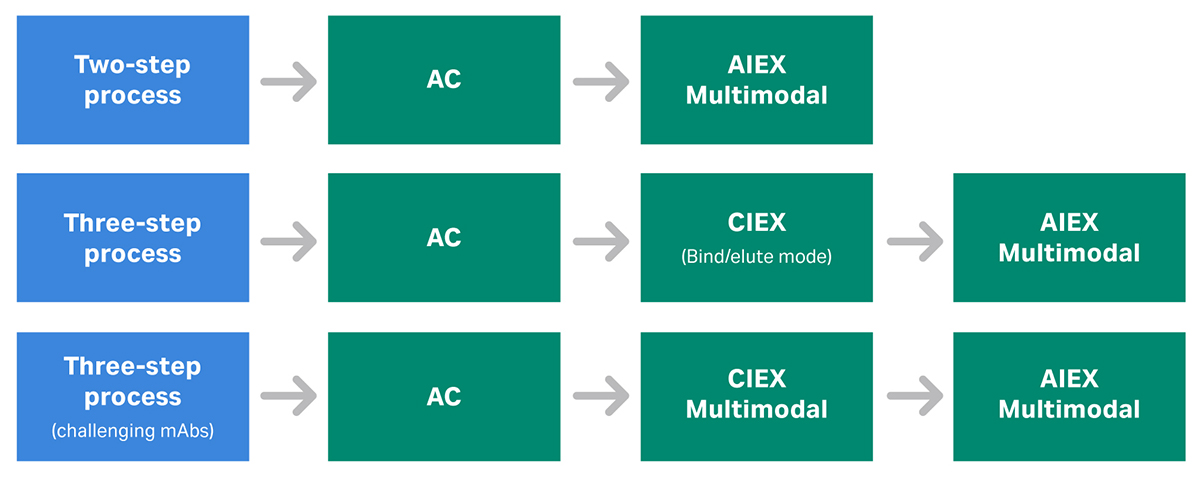
You may need to adapt the purification scheme presented in Figure 2 for some mAbs. Use an MM AIEX step for better performance, or if the antibody to be purified is not compatible with the pH of the AIEX resin (Fig 3).
Fig 3. Examples of chromatography purification platforms involving multimodal (mixed mode) chromatography steps. Here, the order of the polishing steps is interchangeable.
About mAb polishing with HIC
A previous patent for the use of HIC for mAb purification after a protein A capture step has now expired, which now allows HIC in process development of mAb polishing. HIC enables efficient aggregate removal. At high conductivity, HIC also allows viral clearance.
Biosimilars
For biosimilars, speed to market is even more important. Manufacturers of biosimilars therefore try to limit the number of clinical trials. To do so, process developers (and analytical teams) need to prove biosimilarity of, for example, charge variant profiles compared to the original biopharmaceutical.
The upstream process usually differs from the one used for the originator product (different cell lines), which usually generates a different impurity pattern, that is, glycan and charge profiles. The glycan profile is handled during the upstream process while the downstream process must take the charge variant profile into account. If the charge variant profile differs from the originator’s product profile, extra optimization work may be needed in the polishing step, potentially leading to a loss in yield. DoE and mechanistic modeling methodologies can be of great help here as well.
Platforms don’t always apply to novel molecules
In the best case, the polishing step should be incorporated in a platform approach, which means that it should be applicable for a variety of drugs with limited adaptation. However, today’s pipelines produce more complex and diversified molecules. For some of these new molecules, the platform approach does not meet all the requirements and needs to be adapted. So rather than a single purification platform, we’re likely to see toolboxes for different design variants.
Challenging polishing separations
A few examples of challenging separations are described below.
Low protein stability
The type of molecule that needs to be purified and its overall stability has bearing on the polishing approach and the downstream process used. Certain proteins do not tolerate low pH, for example, when eluting from the AC step or at a pH close to the protein’s isoelectric point where aggregates may form. Another example is that that deamidation of proteins may occur at high pH. This may limit the choice of chromatography resins and makes selection of the right resin even more important.
Removal of product-related impurities from antibody-derived molecules
Due to the increasing diversification of mAb-like molecules, like bispecific antibodies and other antibody-derived molecules, the removal of product-related impurities is one of the main challenges.
In the case of bsAb, the expression of a four-chain antibody may lead to production of more than 10 different antibody biproducts, which are very similar and consist of mispairs/homodimers, or half antibodies, and light chain mono- and dimers. Only one of these is the desired molecule while the others are unwanted.
One of the drawbacks of the protein A affinity chromatography step used for capture is that all molecular variants, including mispairs/ homodimers, or half antibodies will bind through the interaction with the FC part of the molecule. Therefore, these molecular variants won’t be separated from the target molecule in many cases. Some selectivity may be achieved during the capture step through the VH3 interaction possible for some VH3 sequences if using MabSelect™ or MabSelect™ PrismA resin.
Also, you may have to try alternative affinity resins for capture such as protein L or use novel recombinant affinity ligands to solve the problem. Protein L may be used if the molecule contains one kappa-light chain for example. However, to distinguish between a mispaired bsAb, only one of the half antibodies should contain this class of chain.
So, while some actions can be taken for the capture step to remove product-related impurities, the choice of polishing resin and process conditions is critical to ensure good removal. MM IEX is sometimes helpful to address this challenge.
This article gives more insights about bispecific antibody purification.
Removal of endotoxin from antibody fragments (Fabs)
Thanks to their non-glycosylated form, Fabs can be produced in microbial cells, rather than mammalian cells. When Fabs are produced in E. coli, host cell endotoxins are important impurities to remove. Even if a large proportion of the endotoxins are removed during the capture step, the polishing process is essential to reach the expected endotoxin reduction. This can be handled downstream with, for example, an AIEX resin or MM resin such as Capto™ adhere resin. Note that using a capture resin that tolerates high concentrations of NaOH (such as MabSelect™ PrismA resin in case the product has VH3 interaction) enables efficient cleaning to remove endotoxins.
This article gives more insights about antibody fragment purification.
Polishing of antibody drug conjugates (ADC)
By hooking up a payload (a highly toxic chemical) to an antibody, specific cells can be targeted. Here, antibodies act to reduce the action of the toxic agent on nontargeted cells, minimizing side effects. The targeted delivery of these cytotoxic agents (payloads) gives them a promising position in oncology research.
An important challenge during downstream purification is to remove the unconjugated antibody, the free cytotoxic drug, and to obtain a drug-antibody ratio (DAR) that matches with the specifications. Furthermore, the size and hydrophobicity of the payload can generate aggregates of the ADC.
The small size of the payloads and small corresponding selectivity changes make removal of these impurities challenging with conventional chromatography resins. The bulk of unconjugated payload is typically removed by ultrafiltration. HIC has been successful in polishing of remaining payload impurities. However, MM could be further explored.
An important consideration for the overall process is to work in closed systems, due to the high toxicity of the molecules. Single-use systems might be of great value here.
Viral clearance
Even if there are process steps that are dedicated for virus inactivation or removal (e.g., virus filtration, pH treatment, or solvent detergent), chromatography steps are expected to contribute to the viral clearance.
To obtain the expected viral log reduction during a purification workflow, additional chromatography steps combined in an orthogonal manner may be required, potentially ending up with a with 4-step process. To prevent this, you should select a resin that works both for obtaining high product quality and viral clearance at the same time.
Some considerations:
- Virus clearance is typically seen at low conductivity in AIEX chromatography
- Virus clearance is typically seen at high conductivity in HIC
- Viral clearance using MM AIEX chromatography is effective at broader ranges of conductivity. For example, Capto™ adhere ImpRes and Capto™ adhere resins have demonstrated good viral clearance
Considerations for making polishing more efficient and straightforward
Benefits of flow-through polishing steps
In flow-through mode, the target molecule will not bind but will rather flow through or weakly interact with the resin, leaving most impurities bound. This is achieved by selecting the conditions (amount loaded, pH and/or conductivity of the load and buffer, or salt) to modify the charge or hydrophobicity of the target molecule or the chromatography resin. The advantages of flow-through mode are that higher load levels can be used, potentially reducing the number of purification cycles, reducing washing needs, and removing elution steps — you only need to be concerned with maximizing yield and binding of impurities.
The purity of the protein of interest found in the flowthrough fractions can be increased by optimizing conditions such as pH, buffer, and salt. A wash step can be used to increase the yield of the target protein by allowing collection of weakly bound target molecule. An elution step is sometimes used to remove/elute some bound impurities before the cleaning-in-place (CIP) step is applied.
Straight through processing (STP)
Downstream chromatography purification processes are traditionally performed as batch processes using hold-up tanks between the different unit operations. The separate chromatography steps, each optimized in terms of buffer substance, pH, and conductivity, often require intermediate conditioning steps.
To remove or minimize the need for hold-up tanks and batch conditioning steps, an STP concept can be applied. STP is when two or more chromatography steps are connected in series, with or without in-line adjustment of process conditions between columns to ensure optimized performance of the next step. To omit the need for intermediate conditioning steps, elution conditions from the previous step must be selected to match the loading conditions for the subsequent step.
Possible resin variability impact on the outcome of polishing process
Sometimes, the inherent variability of a raw material within a manufacturer’s given specification range can affect process outcomes such as purity, yield or productivity. Such cases are more common if there is an interplay of the effect of variability in raw materials and process parameters. In the case of resin ligand density, this is especially true for challenging purifications. Resin variability has been identified to be more likely to affect processes using HIC and MM techniques compared to IEX, and processes using bind/elute mode compared to flow-through mode. This can be overcome with thorough process characterization and an appropriate control strategy. However, it should be stated that the vast majority of chromatography steps are inherently robust to small variability in resin properties.
Importance of resin choice
In contrast to capture purification where a fast, high-capacity step elution is most used, a polishing purification will focus on achieving high resolution, resulting in high purity. Polishing purification is therefore performed using chromatography resins with smaller average particle sizes to increase resolution. However, the reduction in particle size leads to higher back pressures.
Modern resins such as our range of Capto™ resins showcase increased rigidity compared to the earlier, Sepharose based resins, enabling higher flow rates and increased flexibility in bed heights.
Further reading:
- Basics of process development for biotherapeutics
- How to select a chromatography resin
- Bispecific antibody purification: insights and case studies
- Improve Efficiency in mAbs Purification
- Mechanistic modeling of chromatography: opportunities and challenges
- Risk assessment: studying chromatography resin variability
- Increasing productivity in hydrophobic interaction chromatography (HIC) using Capto™ resins
- Future-proof your HCP strategy
IEX resins
- Capto™ DEAE resin
- Capto™ Q resin
- Capto™ Q ImpRes resin
- Capto™ S resin
- Capto™ S ImpAct resin
- Capto™ SP ImpRes resin
- Capto™ Q XP resins
Multimodal (mixed mode) chromatography resins
- Capto™ adhere resin
- Capto™ adhere ImpRes resin
- Capto™ Core 400 resin
- Capto™ Core 700 resin
- Capto™ MMC resin
- Capto™ MMC ImpRes resin
HIC resins
- Capto™ Butyl resin
- Capto™ Butyl ImpRes resin
- Capto™ Phenyl resin
- Capto™ Phenyl ImpRes resin
- Capto™ PlasmidSelect resin
- Capto™ Octyl resin
References
- Biopharmaceutical Processing: Development, Design, and Implementation of Manufacturing Processes”. Eds. Gunter Jagschies, Eva Lindskog, Karol Lacki Parrish Galliher. Elsevier (2018).