Platform approach to purification of bacterial capsular polysaccharides for vaccine production
Capsular polysaccharides (CPS) of encapsulated bacterial pathogens can give rise to an effective immune response in humans, and are commonly used in vaccine production. This article offers an overview of modern tools and technologies that can facilitate CPS-based vaccine production. An alternative purification approach based on chromatography, replacing many of the ethanol and phenol extraction steps of the traditional process, is also presented. Using the proposed purification platform, 28 different CPS of three different species could be processed to high purity and yield in a secure and environmentally friendly way.
Introduction
The growth rate in vaccines looks promising, with a total market worth of 30 billion USD in 2016 and estimated to reach 45 billion USD in 2022 (1). As of 2016, there were more than 250 vaccines in development of US based companies or foreign companies conducting clinical trials in the USA (2). Of the vaccines in development against infectious diseases, about 25 could be attributed to diseases caused by bacteria. In the vaccine pipeline for bacterial diseases, vaccines against Neisseria meningitidis, Streptococcus pneumoniae, and Haemophilus influenza could be found. Capsular polysaccharides (CPS) are the primary cause of the virulence of these bacteria, and are often used in production of vaccines against these pathogens.
As vaccines are often distributed by national ministries of public health, development and production costs need to be low to enable a global reach of the vaccines, especially in emerging markets. Traditionally, CPS purification is performed by extractions, using solvents such as ethanol and phenol to remove nucleic acids and protein, followed by ethanol precipitation and centrifugation to remove endotoxins. For the Gram-negative bacteria S. pneumoniae and H. influenza, an ultracentrifugation step is commonly included to remove lipopolysaccharides. This purification strategy is both time- and resource-consuming. The extractions are often carried out in explosion-proof rooms as the flammability of the required solvents is high, and ultracentrifugation is costly and not all manufacturing facilities have access to such equipment for manufacturing (3). In addition, handling and recycling of the toxic solvents and other components constitute safety and environmental risks. Such a process is also difficult to scale to meet market demands.
Replacement of these traditional purification strategies can help enhance productivity and result in more cost-efficient and more easily scaled processes. A simple platform approach to purification of CPS can contribute to more rapid process development to reduce time to market.
CPS production for vaccine manufacturing
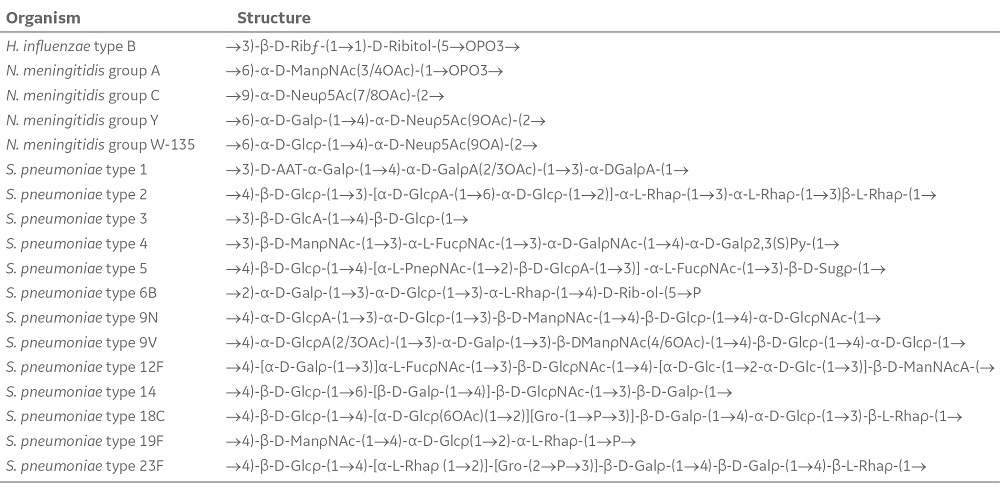
CPS, composed of monosaccharide units joined together through glycosidic and phosphodiester linkages, constitute a highly diverse group of polymers that surround both Gram-positive (e.g., N. meningitidis) and Gram-negative (e.g., S. pneumoniae and H. influenza) bacteria. Of the 13 clinically significant N. meningitidis serotypes, classified according to the structure of their CPS, the serotypes A, C, Y, and W-135 are responsible for 90% of all global cases. For S. pneumoniae, only a few of the 90 different serotypes leads to diseases in humans. H. influenza can occur in both unencapsulated and encapsulated form. There are six types of encapsulated strains (a, b, c, d, e, and f), of which type b is the most familiar. For encapsulated pathogenic bacteria, the concealment of extracellular proteins has prevented the development of protein-based vaccines. However, CPS has shown to give rise to an effective immune response in humans and thus are commonly used for vaccine production (Table 1).
CPS vaccines can be based on purified CPS alone, so called, naked vaccine. More commonly, the CPS is covalently coupled to a carrier antigen in, what is called, a conjugate vaccine. CPS-based vaccines can also be multivalent, where multiple strain-specific components are included to protect against the most prevalent pathogenic serotypes. When looking at the breadth of disease causing strains across the world, it is forecasted that multivalent conjugate vaccines will constitute more than 50% of the world market in the near future (5).
Table 1. Repeated units of selected bacterial CPS involved in vaccine production (4)
UPSTREAM PRODUCTION
In the industry today, production of CPS by bacterial fermentation is commonly conducted in stainless steel systems. The adoption of single-use technology by biomanufacturers using microbial systems has been limited by the lack of single-use bioreactor systems capable of accommodating the unique requirements of microbial cultures. Bioreactors designed for mammalian culture fall short of meeting bacterial cultivation requirements, for example, high oxygen transfer capacity and increased cooling capacity for efficient temperature control.
With the advances in single-use technologies, today’s microbial systems are purpose-designed to overcome these limitations, and can fulfill the demands of bacterial fermentation (Fig 1).

Fig 1. Single-use Xcellerex XDR-50 MO and XDR-500 MO fermentor systems together handle working volumes from 25 to 500 L. The systems deliver performance comparable with hard-piped, stainless steel fermentor systems, while eliminating the time-consuming and costly clean-in-place (CIP), steam-in-place (SIP), and cleaning validation procedures. Single-use technology also increases operator safety, as the system is closed and process components that have been in contact with the process material are disposed after use without the need for open handling of the product.
DOWNSTREAM PURIFICATION
The upstream process determines the type and concentration of impurities that the downstream process will need to be capable of removing. Typical impurities in purification of CPS include host cell protein (HCP), host cell DNA (hcDNA), pigments, and other polysaccharides such as cell wall polysaccharides and lipopolysaccharides. The traditional purification process commonly comprises a sample preparation step to separate CPS from cell debris, followed by removal of remaining impurities in several selective precipitation steps involving solvents like ethanol and phenol as well as cationic detergents. Separation of solid from liquid is performed by centrifugation.
To limit the use of flammable and health hazardous solvents, the extraction steps can be greatly reduced by using modern chromatography resins. Multimodal resins, for example, provide multiple modes of actions such as ion exchange, hydrophobic interaction, and hydrogen bonding, enabling efficient removal of impurities such as HCP and nucleotides, often in one single step (Fig 2).
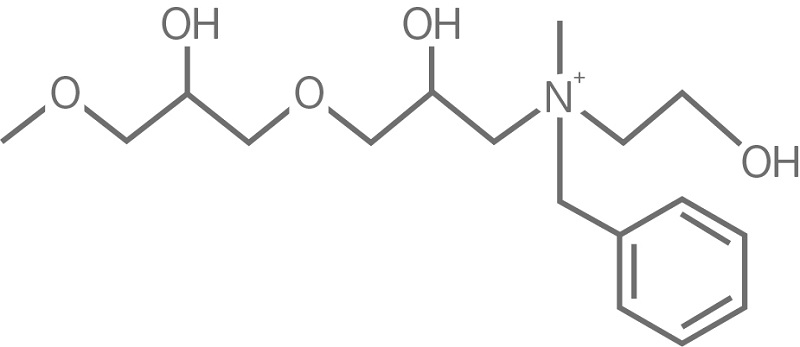
Fig 2. The ligand, N-benzyl-N-methylethanolamine, of Capto adhere multimodal anion exchange resin interacts with the target molecule through multiple modes of action, of which the most pronounced are ionic interaction, hydrogen bonding, and hydrophobic interaction.
The ultracentrifugation step for lipopolysaccharide removal can be replaced by tangential flow filtration (TFF) (6). In TFF, the feed stream moves tangential to the membrane. Particles larger than the pore size are kept in constant movement, avoiding the formation of a filtration cake, which can clog the filter. Hollow fiber filters are commonly used for the TFF step (Fig 3). Because of their open channel structure, hollow fiber filters are well-suited for microfiltration applications like recovery of proteins expressed in bacteria.
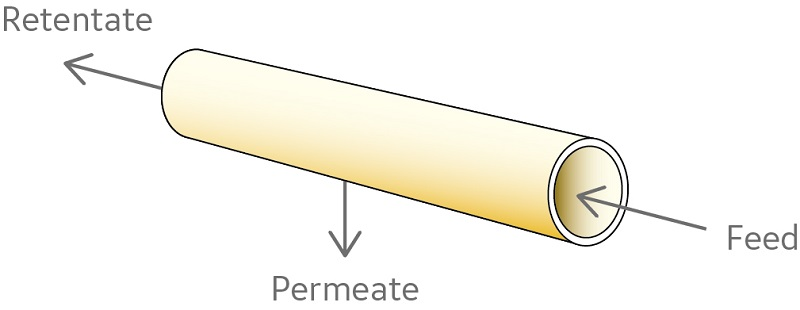
Fig 3. Configuration of hollow fiber filters. The membrane forms a set of parallel hollow fibers. The feed stream passes through the lumen of the fibers and the permeate is collected from outside the fibers. Cartridges are characterized in terms of fiber length, lumen diameter and number of fibers, as well as filter pore size.
Purification techniques such as chromatography and filtration allow fast and easy process development. Design of experiment (DoE) methodologies can be used to perform experiments to identify conditions for maximized productivity (Fig 4). This approach produces maximum amount of data with minimum number of experiments, and meets the demands from regulatory authorities for better process understanding, one of the cornerstones of the quality by design (QbD) initiative. In addition, chromatography and filtration operations are easy to scale and compatible with both single-use and conventional technologies.
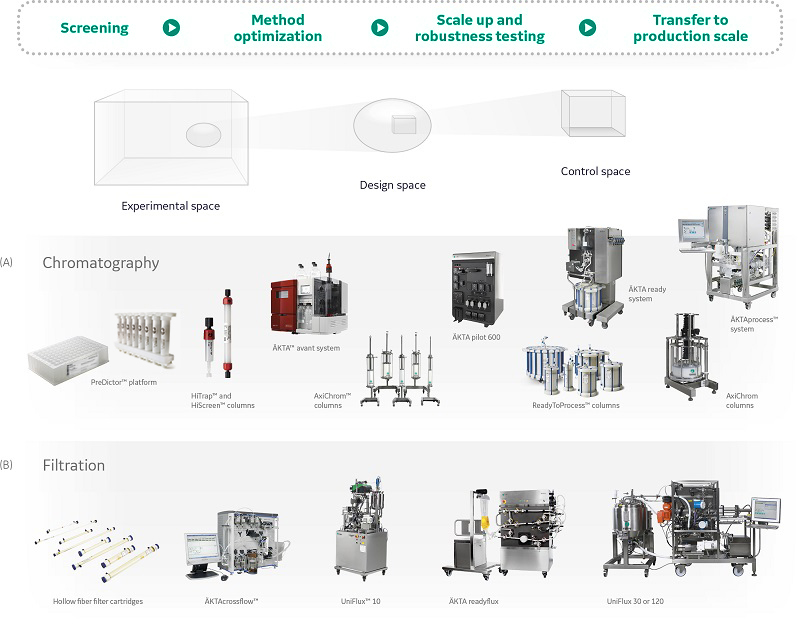
Fig 4. Process development workflow, including initial screening, verification, and further optimization of (A) chromatography and (B) filtration conditions in the purification of a target biomolecule.
Conclusion
This article gives an overview of modern products and technologies that can help solve many challenges in production of bacterial CPS vaccines. Fermentor systems based on single-use technology support significant time-savings, while increasing process- and operator-safety. Process steps involving flammable or health hazardous solvents can be replaced by modern chromatography resins. Cross-flow filtration offers an alternative to costly ultracentrifugation operations. With tools and formats for process development workflows, the time for such activities can be significantly reduced to contribute to an overall shorter time to market. Integrating the different process units for automated operations in a closed system will reduce the number of process steps, thereby minimizing facility footprint. The described purification platform allowed multiple CPS serotypes to be successfully purified with high recovery and purity in a health and environmentally friendly way.
Read more about our vaccine platforms
References
- Carlson, B. For Struggling Pharma Market, Vaccines Offer Path to Revenue. Genetic Engineering Biotechnology News May 23 (2016).
- Report: Medicines in Development for Vaccines 2016. PhRMA August 23 (2016).
- Noyes, A.R. Modular tools for high throughput process development of polysaccharide vaccines. Doctoral thesis, University College London (2015).
- Jones, C. Vaccines based on the cell surface carbohydrates of pathogenic bacteria. An Acad Bras Cienc 77, 293–324 (2005).
- Bae, K., Choi, J., Jang, Y., Ahn, S., Hur, B. Innovative vaccine production technologies: the evolution and value of vaccine production technologies. Arch Pharm Res 32, 465–480 (2009).
- Maimoni Gonçalves, V., Takagi, M., Souza Carmo, T., Barbosa Lima, R., Ferreira Albani, S.M., Ventura Pinto, J., Zangirolami, T.C., de Campos Giordano, R., Massako Tanizaki, M., Cabrera-Crespo, J. Simple and efficient method of bacterial polysaccharides purification for vaccines production using hydrolytic enzymes and tangential flow ultrafiltration. Communicating Current Research and Educational Topics and Trends in Applied Microbiology 1, 450–457 (2007).
- Whitepaper: Platform approach to purification of bacterial capsular polysaccharides for vaccine production. Cytiva, KA2040220118WP (2018)
- Application note: Efficient purification of meningococcal polysaccharides in a twostep chromatography workflow. Cytiva, 29216880, Edition AA (2016).
- Application note: Efficient purification of pneumococcal polysaccharides in a chromatography workflow. Cytiva, 29216881, Edition AA (2016).
- Application note: Efficient purification of Haemophilus influenzae type b capsular polysaccharide in a one-step chromatography workflow. Cytiva, KA2041220118AN (2018).