Challenge
Delivering safe drugs is critical for biomanufacturers. Evaluating how much a process step contributes to viral clearance is therefore an essential part of process validation. For this reason, there is an industry need to perform effective viral clearance studies.
Scope of the viral clearance case study
In this study, the virus reduction of an affinity capture step was evaluated in a scale-down column format. The study was performed by a third-party viral testing laboratory in collaboration with a biopharmaceutical company.
Outcomes
- A robust process providing expected virus removal for different sample loads and residence times.
- Reduction factors were in the typical range for a protein A affinity chromatography step.
- The chromatography column format was well-suited for the viral clearance study.
Why evaluate viral clearance capacity and tools?
Nearly all commercially approved mAb manufacturing processes use protein A capture as the initial step in downstream purification. This chromatography step is not dedicated to removing virus particles. Rather it is contributing to viral clearance as the virus flows through the column, while the target mAb is captured by the resin.
When evaluating which capture resin to use, it is central to weigh in its viral clearance capacity. A log reduction factor in the range of around 2.5 is typical for a protein A chromatography step (1). This study evaluated the virus clearance capacity of MabSelect PrismA resin.
A large number of columns are needed for viral clearance studies over a year’s time. To minimize the risk of having to repeat the studies, it is important to make sure the column quality is high. This study used HiScale 10/40 columns.
Materials and methods
Resin, column, and packing parameters
MabSelect PrismA chromatography resin was packed in HiScale 10/40 columns using the following packing parameters:
- Slurry concentration: 50%
- Packing solution: 20% ethanol with 0.4 M sodium chloride
- Settling velocity: 200 cm/h
The resin was compressed with packing factor 1.10 to a final bed height of 20 cm. The packed bed was conditioned with a flow velocity of 400 cm/h during 10 min.
Viruses used in the viral clearance study
Two different model viruses were tested, which is typical for early phase studies of Chinese hamster ovary (CHO)-based expression systems. For late phase studies, four viruses and duplicate studies are required. (2)
The model viruses used were Xenotropic murine leukemia virus (xMuLV) and Minute virus of mice (MVM) (Table 1). The titer of xMuLV was analyzed by q-PCR. MVM titer was analyzed in a bioassay.
Table 1: Overview of the two virus types used in the viral clearance study
| Virus | Family | Genome | Size (nm) | Enveloped | Resistance to physical and chemical treatments |
|---|---|---|---|---|---|
| xMuLV | Retro | ss-RNA | 80–110 | Yes | Low |
| MVM | Parvo | ss-DNA | 18–24 | No | Very high |
ss = single-stranded
Design of experiments to challenge viral clearance robustness
To challenge the method robustness, different scenarios were studied where the sample load and residence time (RT) varied (Table 2). The standard set up was run in duplicate. The resin bed height and column volume were approximately 20 cm and 16 mL, respectively.
Table 2. Details of the different scenarios studied
| Scenario | Sample load conc. (mg/mL) | RT in chromatography process (min) | RT in loading step (min) |
|---|---|---|---|
| Standard 1 | 65 | 6 | 12 |
| Standard 2 | 65 | 6 | 12 |
| Low sample load |
20 |
6 |
12 |
| Short residence time |
65 |
4 |
4 |
The following conditions were used for the four scenarios: equilibration with 5 column volumes (CV) PBS, three washes subjecting the columns to 3 CV of PBS, high salt, and acetate, respectively.
Results and discussion
The results from the viral clearance study is summarized in Figure 1. Compared with the standard runs, slightly higher reduction factors for low load conditions and short residence time were observed for xMuLV. The opposite was observed for MVM.
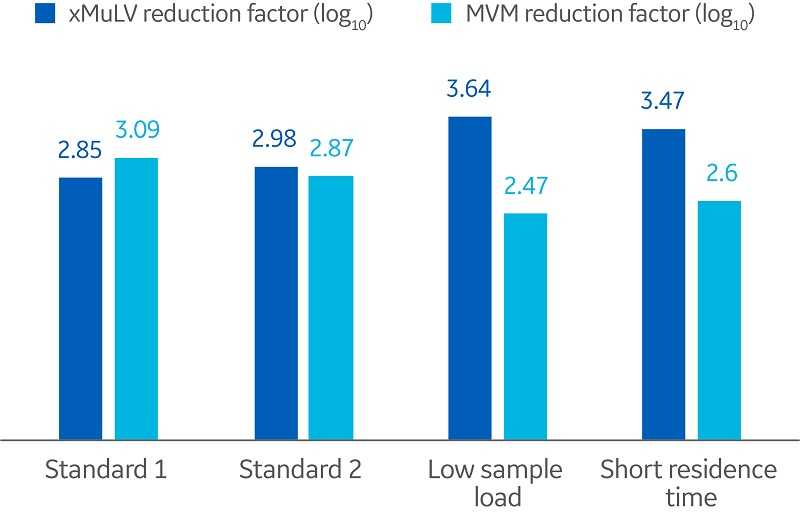
Fig 1. Summary of reduction factor results in relation to sample load and RT.
The difference in results for the two virus types indicates that the interaction between the virus particle and the mAb or the resin is different under the given process parameters. However, a larger study would be required to further investigate this.
Conclusion: reduction factors within the typical range
Overall, the reduction factors observed in this study were within the typical range found for protein A resins. This demonstrates expected virus removal and a robust process even when sample load and residence time are varied.
This study shows that HiScale 10/40, packed with MabSelect PrismA, is a reliable choice for the capture step in a virus clearance study. Together, the column and resin offer good viral log reduction in a suitable scale-down column format.
You can find the column specification details on the HiScale 10/40 column product page.
References
- Ruppach, H. Log10 Reduction Factors in Viral Clearance Studies, BioProcess. J., 12(4), 24–30 [Online] https://www.bioprocessingjournal.com/ (7 January 2014 posting date)
- Viral Safety Evaluation of Biotechnology Products Derived from Cell Lines of Human or Animal Origin, ICH, Q5A (R1), (1999)