By Ryan Zolyomi, Ian Scanlon*, Florence Rusly, Oliver Hardick*, Ashley Hesslein, and Hendri Tjandra.
Biological Development – Isolation & Purification, Bayer U.S. LLC, Pharmaceuticals and Cytiva*
In clinical monoclonal antibody (mAb) manufacturing, capture on protein A chromatography resin gives excellent recovery and purity. However, because clinical batch sizes are small, fewer cycles are required compared with full-scale manufacturing; this means that the full resin lifetime is rarely realized. Bayer worked with Cytiva to evaluate a fiber-based alternative, Fibro PrismA, to determine its potential as a truly single-use mAb capture technology. We assessed scalability from lab- to process-scale units, tracking pressure drop, step recovery, purity, and eluate volumes across multiple cycles.
Compared with a protein A resin-based process, initial process modeling of Fibro chromatography suggests substantial gains in productivity with somewhat higher buffer consumption and eluate volumes. Those volumes could be minimized further through optimization if required for facility fit. The utility of Fibro PrismA is therefore suitable for product commercialization.
Evaluating fit within our existing platform
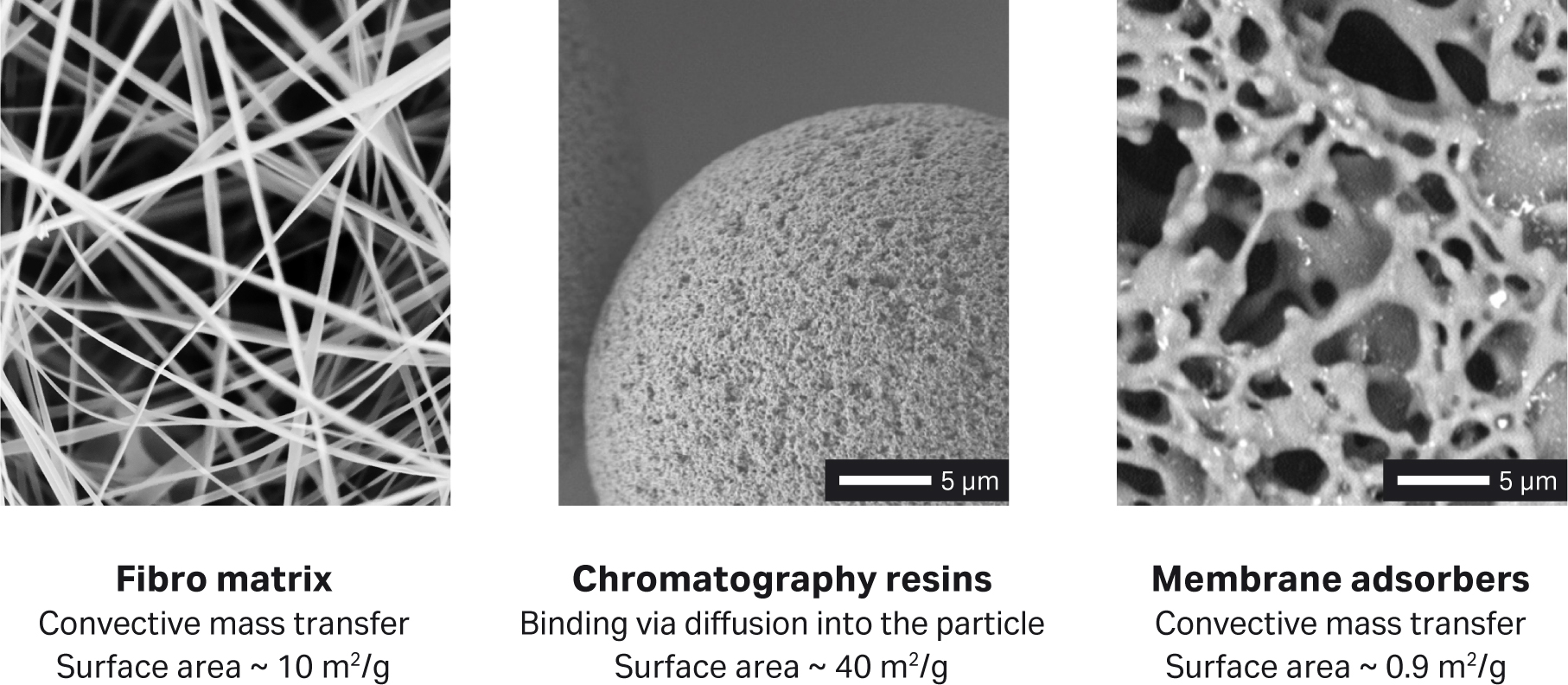
In our current mAb platform process, chromatography resins are the primary barrier to having a fully single-use process, which would offer easy and quick change-over between campaigns. Such a process would also provide operational flexibility in a multi-product manufacturing facility. With these goals in mind, Cytiva’s Fibro PrismA chromatography for the capture step was evaluated. Fibro chromatography uses a well-defined matrix of cellulose fibers that has a very open structure relative to chromatography beads or membrane adsorbers. The proprietary structure allows the technology to overcome the diffusional and flow limitations of packed bed chromatography purification, as well as the capacity issues of membrane adsorbers (see Fig 1).
Fig 1. Surface area and mass transfer mechanisms for different chromatography base matrices.
One of the drivers to consider with Fibro technology is the high number of cycles possible within a day of operation, which allows the full lifetime the Fibro unit to be realized in a single batch. Particularly in situations where product turnover or small batches are needed, this substantially improves the efficiency of single-use chromatography in clinical and commercial manufacturing by eliminating column packing, cleaning validation, and storage of columns.
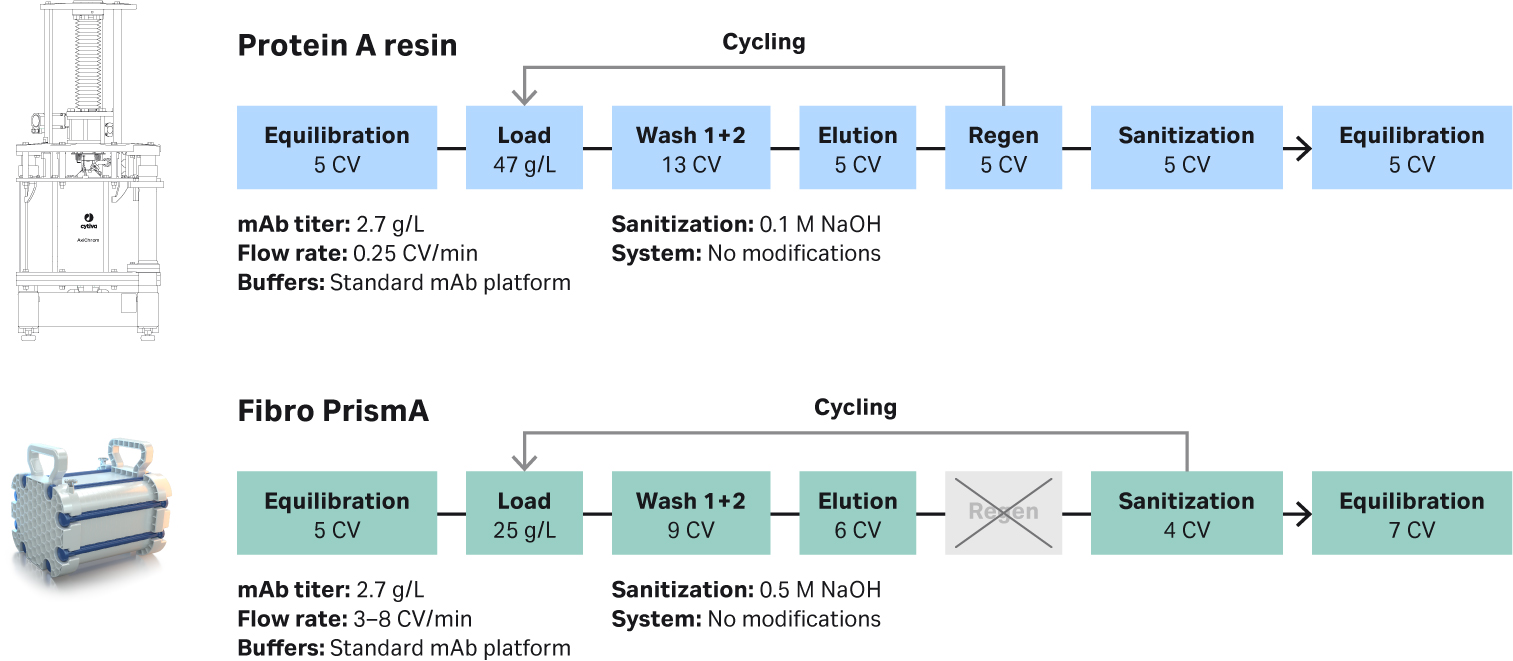
We designed a set of experiments to evaluate lifetime and scalability of different sized Fibro PrismA units from bench scale to process scale using standard scale-up parameters. The platform process we developed is shown in Figure 2. Lab experiments demonstrated that cycling time for Fibro PrismA can be optimized by decreasing the CVs and eliminating the column regeneration step. The flow rate for Fibro PrismA is significantly faster than a typical protein A resin-based process. Flow rates can be limited by the system hardware (e.g. chromatography skids) rather than the Fibro device flow constraints. With the Fibro units a full chromatography run can be completed relatively quickly, which allows rapid cycling of the units to purify the same volume of load material.
Fig 2. Comparison of mAb platform purification using (top) a protein A resin-based process with Cytiva’s MabSelect SuRe™ LX; and (bottom) Fibro PrismA process.
We distributed 500 L of clarified cell culture fluid (CCCF) from a mAb to the runs listed in Table 1.
Table 1. Fibro PrismA evaluated from bench to process scale
| HiTrap™ Fibro PrismA (0.4 mL) | HiScreen™ Fibro PrismA (3.75 mL) | Pilot Fibro PrismA (40 mL) | Large Pilot Fibro PrismA (160 mL) | Process Fibro PrismA (2.4 L) | |
| Cycle time | 3.5 min | 4.5 min | 4.5 min | 12 min | 12 min |
| mAb load/cycle | ~ 12 mg | ~ 90 mg | ~ 1 g | ~ 4 g | ~ 55 g |
| Cycles | 250 | 200 | 200 | 101 | 16 |
| Chromatography system | ÄKTA™ avant 25 | ÄKTA™ avant 150 | ÄKTA™ pilot 600 | ÄKTA ready | ÄKTA ready |
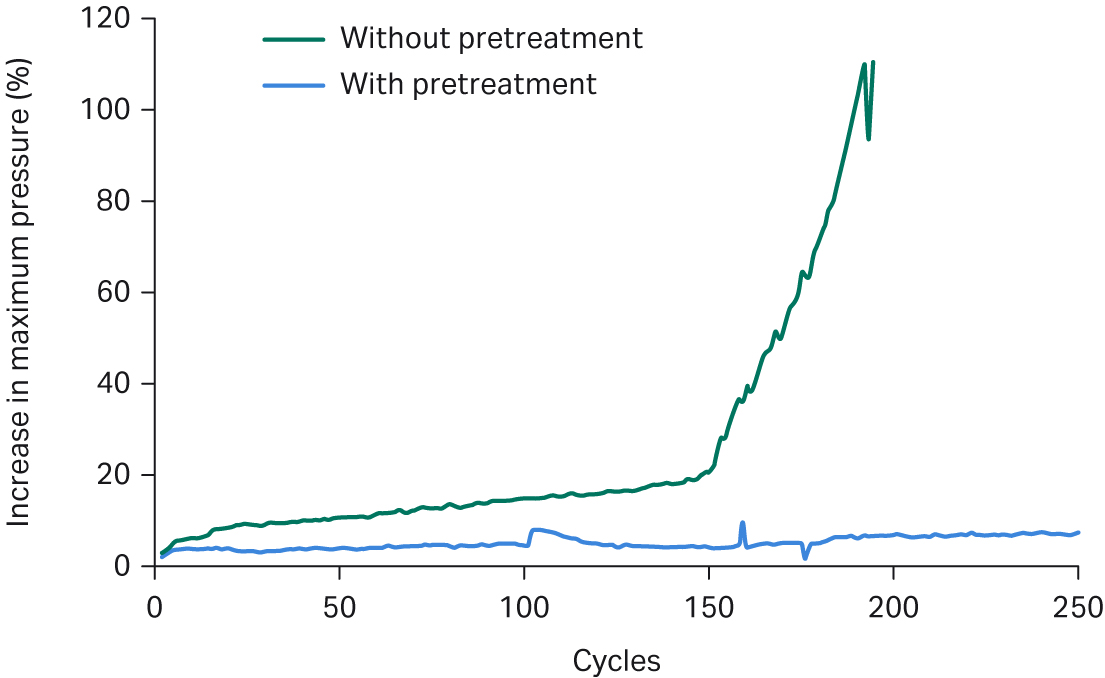
The clarified harvest was further filtered prior to loading on the Fibro PrismA units. Our studies showed that this additional filtration increased the lifetime of the Fibro units by up to 40% by minimizing unit fouling and pressure increase over multiple cycles (see Fig 3). While lifetime studies performed on a resin-based process require significant time and resources, it took less than 24 hours to perform lifetime studies on both feed streams on the Fibro PrismA units. We were able to quickly demonstrate that the additional filtration step extended the unit lifetime dramatically by substantially reducing the pressure increase over cycles.
Fig 3. Cycling studies on Fibro PrismA units with or without filtration before loading.
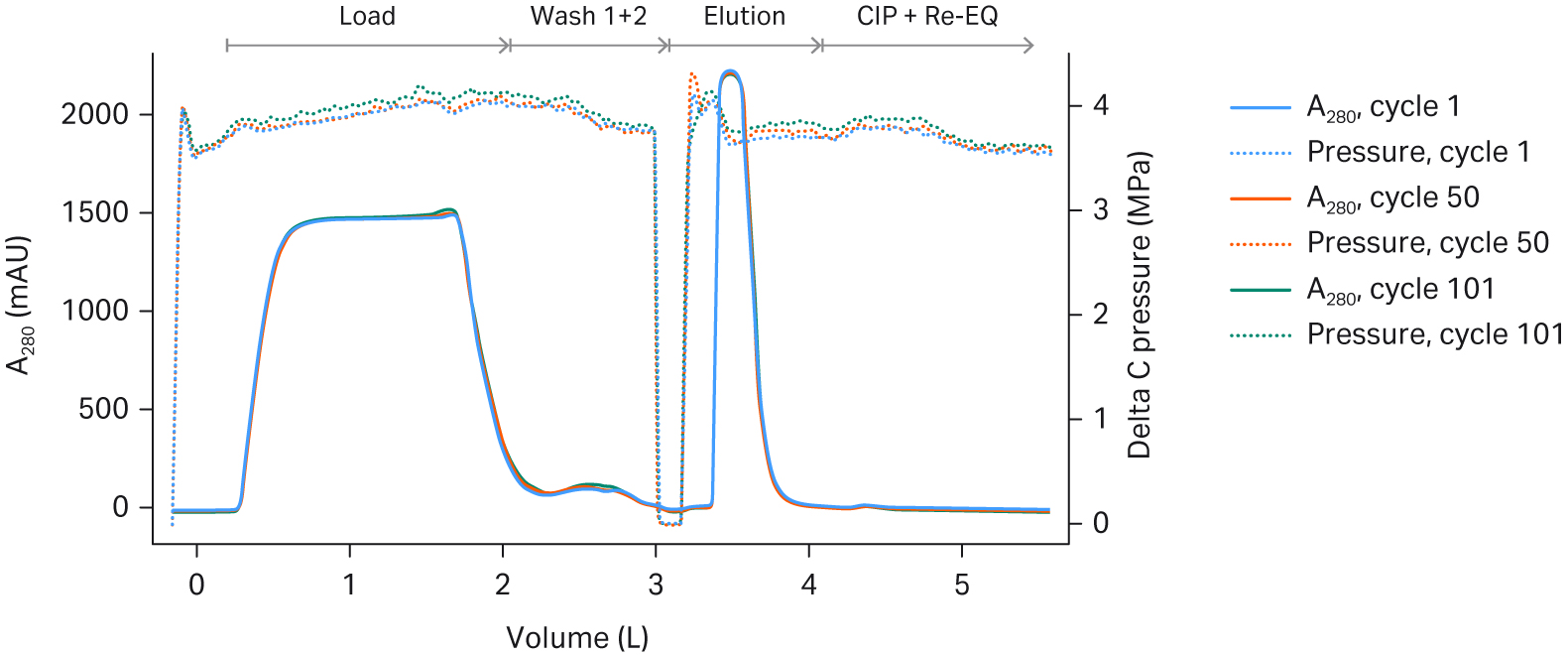
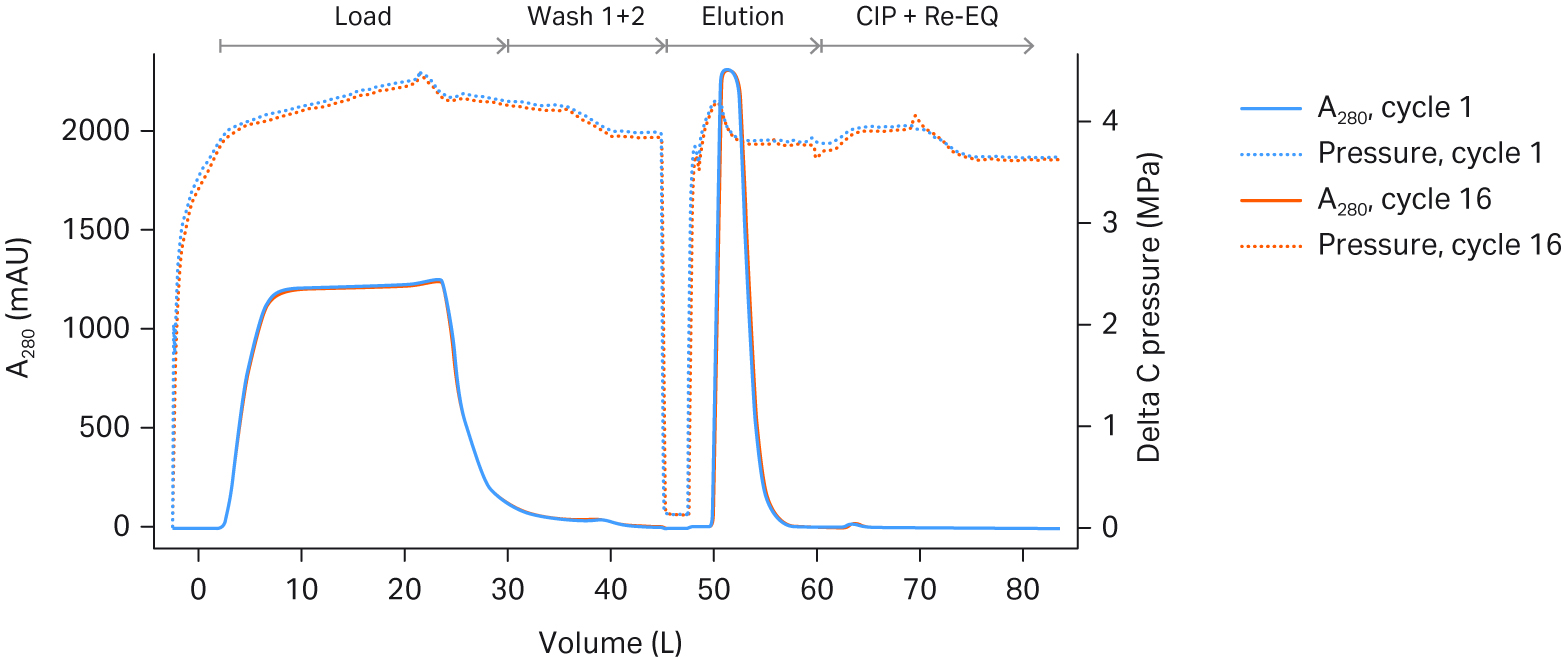
In this study, we evaluated the single-use skid for the 160 mL Pilot unit and the 2.4 L Process unit. These unit sizes are appropriate for 50-200L and 500-2000L bioreactor scale, respectively. The cycle time was dictated by pressure limitations on the single-use chromatography system. The process unit was run at the maximum flow rate for the single-use ÄKTA™ ready High Flow Kit. Delta pressure across both units rose slightly over the cycles, but it was within acceptable limits (see Fig 4).
A)
B)
Fig 4. UV 280 and pressure (DeltaC pressure) overlays across cycles on Fibro PrismA (A) 160 mL Pilot unit; and (B) 2.4 L Process unit.
Evaluating scalability and product quality
While processing, we encountered some issues that led to non-uniform eluate pooling across scales. These issues were related to timing, accidental introduction of air, and uneven cycle numbers between units. Table 2 shows the cycle numbers that correspond to each pool.
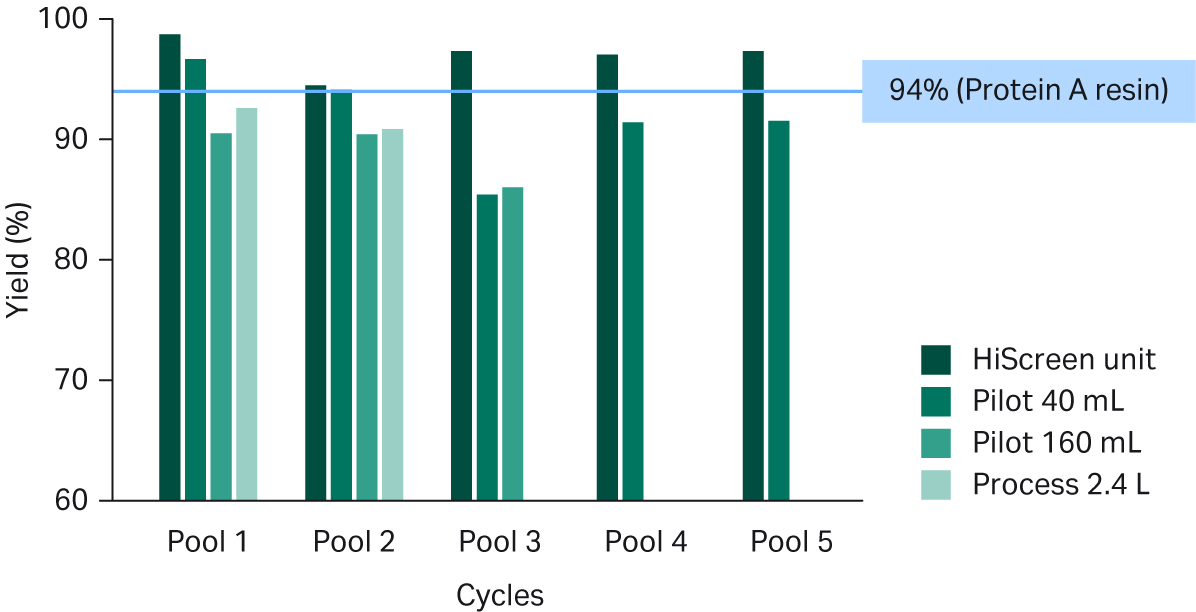
Overall, the recovery was comparable to a standard resin-based process, with greater than 90% yield in most cases (see Fig 5).
Table 2. Fibro PrismA evaluated from bench to process scale
| Pool 1 | Pool 2 | Pool 3 | Pool 4 | Pool 5 | |
| HiScreen™ unit (3.7 mL) | Cycle 1 | Cycle 2‒33 | Cycle 34‒175 | Cycle 176‒199 | Cycle 200 |
| Pilot 40 mL | Cycle 1 | Cycle 2‒100 | Cycle 101‒127† | Cycle 128‒199 | Cycle 200 |
| Pilot 160 mL | Cycle 3 | Cycle 4‒100 | Cycle 101 | - | - |
| Process 2.4 L | Cycle 1 | Cycle 2‒16 | - | - | - |
* Future cycling studies will focus on pooling the cycles in a consistent manner.
† Accidental introduction of air.
Fig 5. Recovery of pooled eluates from Fibro PrismA units of different scales. Recovery of the standard MabSelect SuRe™ LX resin process is 94%, as indicated by the blue line.
We observed a slight downward trend on the Pilot units, which could have resulted from to the slightly different ligand coupling process on these prototype units. This difference is not expected to be present in the final released Fibro units. In addition, recovery from Pool 3 from the smaller Pilot unit was lower because air was accidentally drawn into the Fibro unit prior to elution, which perturbed UV-based collection of eluates. In a follow-up study, we optimized the UV collection criteria on the ÄKTA™ system’s UNICORN™ software, and recoveries were consistently > 90% for all unit sizes (data not shown).
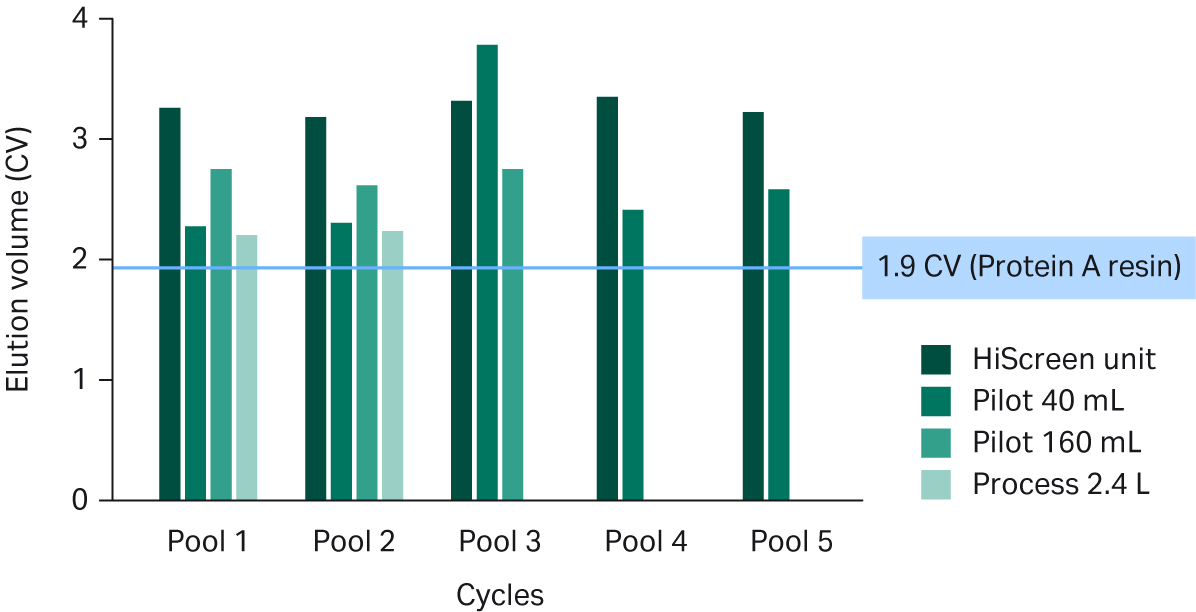
When assessing scalability, it is important to measure the elution volumes across unit sizes. However, the elution volumes are also tied to the system hold-up volume relative to the Fibro unit volume. As shown in Figure 6, the Pilot and Process units produced elution volumes of < 3 CVs. The elution pool volume has been optimized on the Pilot and the Process Fibro units to give a more concentrated elution pool compared to the HiScreen™ Fibro PrismA unit. The larger pool volumes for the HiScreen™ Fibro PrismA units are expected.
The eluate volumes from the Fibro units may be a limiting factor in some facilities, especially when the eluates from the protein A step are pH adjusted.
Fig 6. Elution volume (CV) of pooled eluates from Fibro PrismA units of different scales. Elution volume of the standard MabSelect SuRe™ LX resin process is 1.9 CV, as indicated by the blue line. The larger elution volume for pool 3 on the 40 mL Pilot unit was caused by accidental introduction of air in the unit prior to elution.
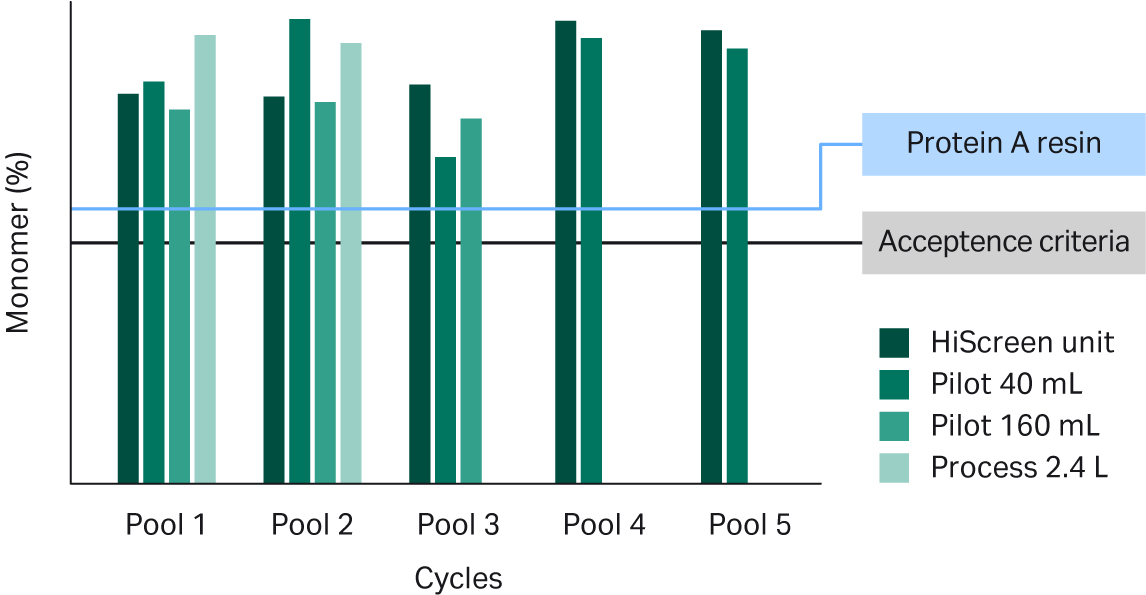
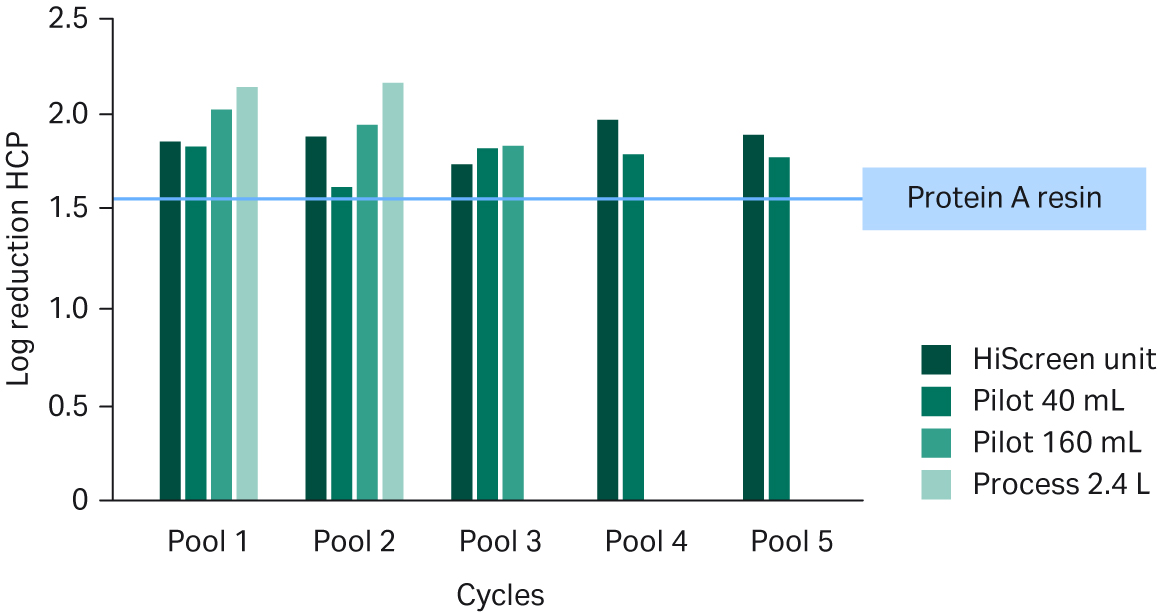
We also looked at the product- and process-related impurities in the Fibro eluate. The product monomer levels in the eluate generated by all the units and pools were above the levels typically requested by regulatory agencies and were comparable to results obtained with MabSelect SuRe™ LX resin (see Fig 7). Host cell protein (HCP) reduction may present extra challenges in cases where mAb candidates co-purify with HCPs such as the mAb in Figure 8. Nonetheless, the Fibro eluate pools had slightly better HCP reduction compared to the resin-based process (see Fig 8).
Fig 7. Percent monomer of pooled eluates from Fibro PrismA units of different scales. Value for the standard MabSelect SuRe™ LX resin process is indicated by the blue line.
Fig 8. HCP log reduction of pooled eluates from Fibro PrismA units of different scales. Value for the standard MabSelect SuRe™ LX resin process is indicated by the blue line.
Evaluating productivity and facility fit
As shown in Table 3, we list out three scenarios for a 2000 L bioreactor with a mAb titer of 5 g/L: standard resin-based process; experimental Fibro PrismA process; and optimized Fibro PrismA process. The experimental Fibro process represented minimal process changes. The optimized process had an increased loading density and flow rate to decrease time and increase productivity. In all scenarios, sanitization was performed after each cycle.
Table 3. Process modeling assumptions for a 2000 L bioreactor with 5 g/L* mAb titer. Fibro PrismA values are normalized to those of Mab Select SuRe™ LX
| MabSelect SuRe™ LX protein A resin | Fibro PrismA (experimental) | Fibro PrismA (optimized) | |
| Volume of resin/fiber matrix (L) | 1x | 0.08x | 0.08x |
| Loading density (g/L) | 1x | 0.53x | 0.64x |
| Flow rate (CV/min) | 1x | 12.00x | 16.00x |
| Cycles/batch | 1x | 23.86x | 19.86x |
| Buffer consumed (L) | 1x | 1.35x | 1.12x |
| Eluate volume (L) | 1x | 2.07x | 1.73x |
| Core process time (hr) | 1x | 1.43x | 0.92x |
| Productivity (g/L/hr) | 1x | 9.41x | 14.60x |
*5 g/L, which is a convenient number in the mid-range of commercial titers.
As shown in Table 3, when running Fibro at the lower end of the loading range, total buffer consumption is about ~ 35% more volume for the experimental Fibro process compared to that of the resin process. This could present facility-fit issues, such as a facility limitation of 1000 L portable tank. Following the protein A step, a low pH hold is performed, which requires an additional volume for the pH titration steps. The Fibro eluate volume projected for our current experimental setup would push it to the maximum fill volume of such a portable tank. One way to address this issue is by reducing the Fibro eluate volume with single-pass TFF (SPTFF) before low pH hold. However, this would add another layer of operational complexity to the cycling process. Compared with the resin-based process, productivity is at least 9.4 times higher, in terms of g/L of Fibro matrix per hour.
We also modeled the benefits of operating the Fibro unit at the high end of loading density, which has been demonstrated as the 80% dynamic binding capacity (DBC) for several other molecules. Increasing loading capacity by 20% and increasing flow rate by 33% reduces both the buffer consumption and the eluate volume to levels that are more in line with those of the resin process. Furthermore, in the optimized scenario processing times are shorter than those for the resin process and productivity is nearly 15-fold higher, in terms of g/L of Fibro matrix per hour (see Table 3).
Manufacturing scenarios where Fibro PrismA could be a good fit
Based on these studies, Fibro PrismA could be beneficial in clinical manufacturing where the lifetime of resin is rarely, if ever, fully exhausted. Therefore, there is a potential to reduce costs of the protein A process for clinical projects for which only a few batches are produced. Another use for which Fibro is well-suited is for low-volume commercial mAb products where dosing requirements are low or patient population is small. Thus, even in commercial manufacturing, batches that are produced infrequently can still be produced in a cost-effective manner.
There are several advantages to Fibro PrismA over other protein A capture alternatives. First, it is possible to use the same chromatography systems and buffers as the corresponding batch resin processes. Further, it allows the flexibility to prioritize between high utilization or rapid processing. Finally, the process set-up is relatively simple. However, buffer consumption and eluate volumes are higher than for resin-based processes. Users can overcome these trade-offs to some extent. Once they develop ways to minimize the trade-offs, long-term impact is minimal. However, the benefits of not needing to pack columns, perform functional testing, as well as clean and store columns for months for every campaign are substantial. It is difficult to put a tangible cost on these activities.