In this study, the hydrophobicities of newer generation Capto HIC resins were compared to Sepharose High Performance and Sepharose Fast Flow HIC resins. A monoclonal antibody (mAb) and its corresponding antigen-binding fragment (Fab) were loaded on each resin and eluted in a linear salt gradient. The relative hydrophobicity of each resin was determined from the elution volume.
The study generated a complete hydrophobicity map for our hydrophobic interaction chromatography (HIC) resins. Results showed that relative hydrophobicity of a resin is dependent upon sample composition, emphasizing the importance of testing resin hydrophobicity for each biomolecule.
An application example is also presented. Aggregate removal results are compared using Capto Butyl ImpRes and Butyl Sepharose High Performance resins. Capto Butyl ImpRes achieved a similar decrease in aggregate content with the potential to use higher bed heights and increased flow rates.
Introduction to hydrophobic interaction chromatography
Downstream processes for biomolecule purification are becoming more complex. Challenges include increasing titers from upstream cell culture, greater diversity of target molecules, and a need for better productivity. HIC has characteristics designed to solve these challenges (1). Modern HIC resins are needed to meet the demands of higher flow rates and better throughput without compromising performance.
HIC is a biomolecule purification technique that uses differences in hydrophobicity to separate proteins. The strength of the reversible interaction between proteins and the hydrophobic surface of an HIC resin is influenced by the properties of the HIC resin and proteins of interest, as well as salt concentration. It is, therefore, important to screen molecules of interest to determine which resin and conditions are the most favorable for separation. In general, HIC is useful for the capture, purification, and polishing steps of protein purification.
Using gradient or step elution with hydrophobic interaction chromatography
HIC processing with a linear gradient elution involves reducing salt concentration linearly. This allows proteins to elute sequentially based on the strength of the hydrophobic interaction with the resin, with the most hydrophobic protein eluting last. This method is effective for four different applications: for resin screening, profiling proteins from a sample of unknown components, achieving high-resolution separation, or determining optimal separation parameters.
A step elution involves separate and distinct additions of elution buffer at decreasing salt concentrations. This process is used once HIC conditions have been optimized, achieving high purity and allowing for faster separation times with reduced buffer consumption.
Due to increased rigidity and cross-linking as compared to earlier generation Sepharose resins, Capto resins allow for higher flow rates and increased throughput. Physical and chemical stability also support cleaning-in-place (CIP), resulting in a less frequent need to repack columns.
Results of the hydrophobicity mapping and aggregate removal assessment
Hydrophobicity mapping for HIC resins
In this study, the aim was to generate a hydrophobicity map using a mAb and its corresponding Fab. Gradient runs were performed using a mAb and its corresponding Fab. Elution positions for the samples on the different resins were compared and organized from least to most hydrophobic (Fig 1). A comparison to the previous mapping with model proteins showed that the butyl resins were most prone to change in relative hydrophobicity when comparing model proteins and the mAb (data not shown).
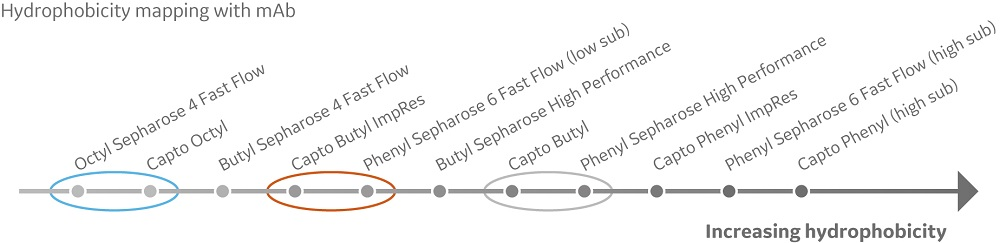
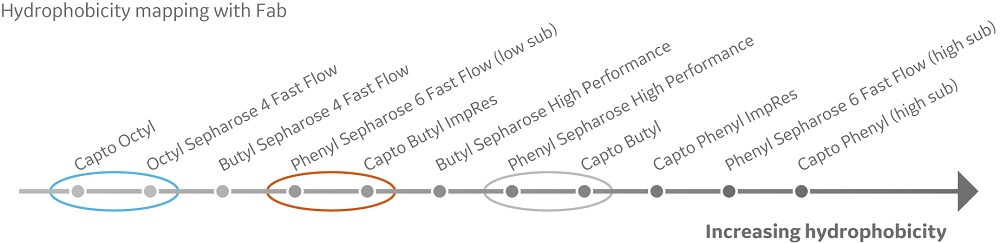
Fig 1. Hydrophobicity scale with a mAb and its corresponding Fab. Increasing hydrophobicity indicates increased binding strength and later elution in the gradient. The circles highlight where hydrophobicity order of the evaluated HIC resins is different between the studied molecules.
Aggregate removal via hydrophobic interaction chromatography
HIC is a valuable technique for removing aggregates. Here, an application example is presented comparing aggregate removal on Capto Butyl ImpRes and Butyl Sepharose High Performance resins (Fig 2–3). While Capto Phenyl ImpRes and Phenyl Sepharose High Performance resins were also initially considered, separation was more difficult to achieve. This suggests that the phenyl resins were too hydrophobic for the mAb in this experiment, and they were not included in this portion of the study.
Fig 2. Capto Butyl ImpRes and Butyl Sepharose High Performance were chosen for the aggregate polishing step due to their small particle size and thus high-resolution properties.
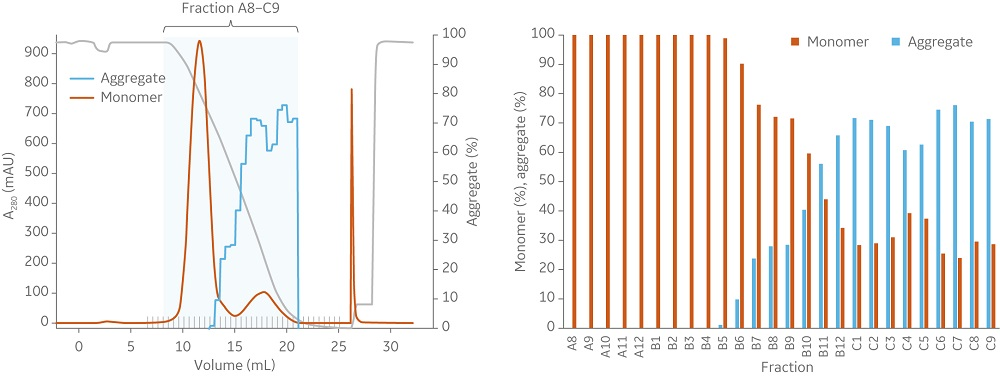
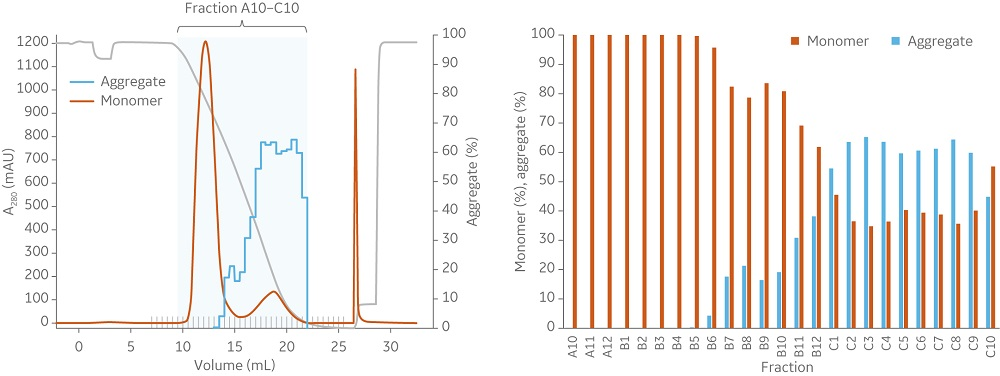
Fig 3. At a load of 66% of QB10 in 1 mL columns (10 cm bed height) with a flow velocity of 75 cm/h, (A) Capto Butyl ImpRes and (B) Butyl Sepharose High Performance both effectively decrease the aggregate content and show similar elution profiles.
Capto Butyl ImpRes and Butyl Sepharose High Performance resins showed similar capabilities in aggregate removal (Figure 3). Using Capto Butyl ImpRes instead of Butyl Sepharose High Performance improves productivity (Figure 4) as higher bed heights and flow rates can be used while maintaining monomer purity and favorable yield (Figures 5–6). Mass balance for the monomer and aggregates was calculated for this final run using Capto Butyl ImpRes at a flow velocity of 200 cm/h and bed height of 20 cm. Results showed an aggregate content below 1% at 98% monomer yield.
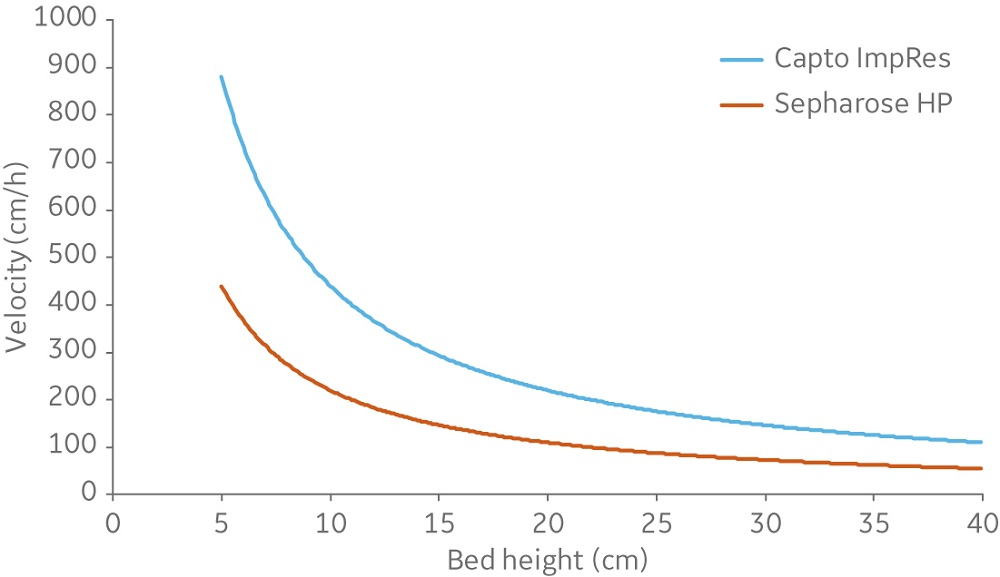
Fig 4. The window of operation (area below the curves) of Sepharose High Performance and Capto ImpRes resins. Data correspond to a process diameter column at 20°C and viscosity equivalent to water.
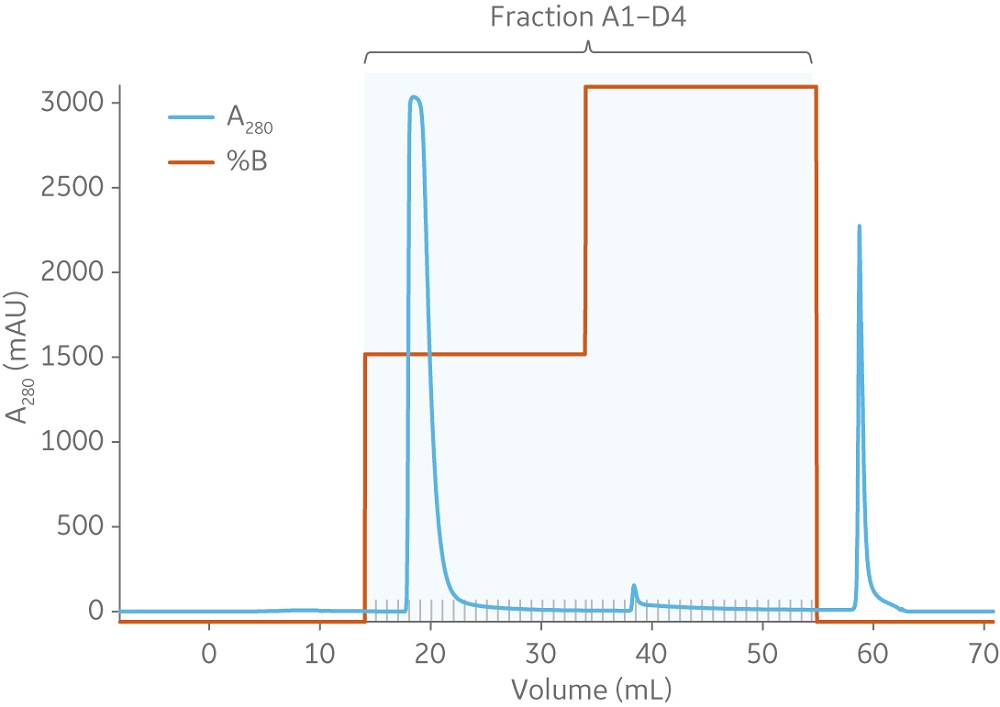
Fig 5. Results from Capto Butyl ImpRes with load of 65% of QB10 for mAb and aggregate mixture show favorable monomer yield with increased flow velocity (200 cm/h) and bed height (20 cm). From left to right, peaks represent the monomer, aggregate, and CIP. The marked fractions were analyzed for aggregate, see Figure 6.
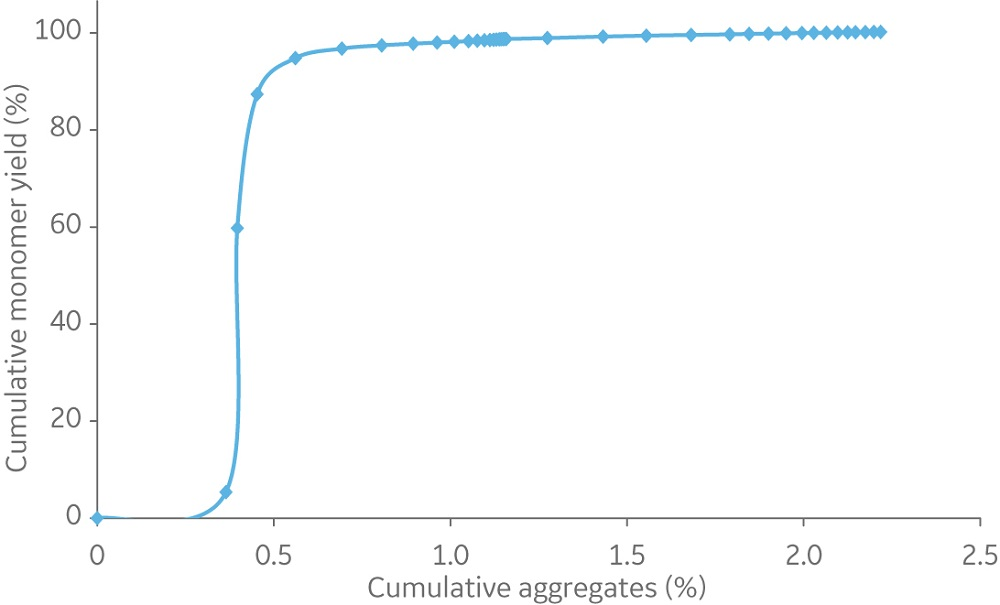
Fig 6. Cumulative monomer yield versus cumulative aggregate content using Capto Butyl ImpRes and a mAb sample containing 10.3% aggregate at 20 cm bed height and a flow velocity of 200 cm/h.
Conclusions
The relative hydrophobicity scale of the resins in this study was different than the hydrophobicity mapping previously performed with other model proteins. The results from this study indicate that the hydrophobicities of the butyl resins were most prone to change based on the sample molecule (i.e., the hydrophobic protein used).
This highlights the importance of screening to find the most suitable resin for the target molecule of interest. The HIC selection kits are available to support choosing the most efficient resin for every specific target molecule.
Aggregate removal to less than 1% was achieved at increased bed height and flow velocity with Capto Butyl ImpRes resin. The chemical and physical stability of Capto HIC resins also support CIP. This allows for less frequent column repacking. These efficiency benefits and the potential for improved productivity make Capto HIC resins a favorable option for high-throughput industrial applications.
References
- Hydrophobic Interaction and Reversed Phase Chromatography – Principles and Methods, Cytiva, 11001269, Edition AA (2006).