Cost-effective purification
Monoclonal antibodies (mAbs) have realized their potential over the last 20 years, with successful applications in a range of major therapeutic areas, including the treatment of breast cancer. In fact, they are the largest and fastest growing class of biological drugs today.
As a result, there is high demand for solutions that will deliver efficient, flexible, and cost-effective mAb purification. Increases in upstream titers mean that downstream processes must gear up too. Higher titers mean a higher number of impurities is likely, and these need to be separated from the target molecule. So what is the most effective way to optimize purification?
Advancing mAb purification using a platform approach
As a class of molecules, mAbs exhibit many shared properties that make them well-suited for a platform approach to downstream processing. Technology platforms allow for efficient processing from research and development, through clinical phase trials, to manufacturing.
Downstream mAb purification platforms commonly include a protein A-based capture step followed by one or two polishing steps to remove remaining impurities. This offers high purity and a high degree of recovery in a single capture step.
Capture using protein A ligands
MabSelect is our protein A affinity family of resins designed for capturing mAbs from large sample volumes. The resins are based on a highly cross-linked agarose matrix with a recombinant protein A ligand. The ligand is derived from E. coli so is free of components of mammalian origin. MabSelect SuRe products have an alkali-stabilized protein A resin. This allows for efficient cleaning and sanitization with cost-effective reagents such as sodium hydroxide, eliminating the need for expensive and hazardous cleaning agents.
MabSelect SuRe LX was introduced to meet the needs associated with high-titer upstream processes. This resin show an increased dynamic binding capacity (DBC) at a slightly longer residence time compared with MabSelect SuRe. It offers better economy at scale and its generic elution profile for different mAbs enables a platform approach to purification.
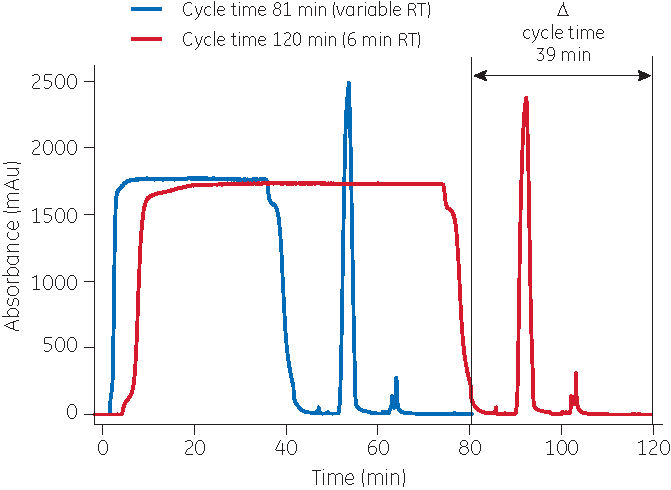
Increased productivity using variable loading
The productivity of a chromatography step can be improved by applying the concept of variable loading. Variable loading means that the loading step is divided into two or more parts.
In a three-step loading procedure for MabSelect SuRe LX, for example, the material is initially loaded at a high flow rate (a short residence time). When 80% of the capacity at 10% breakthrough is reached, the flow rate is decreased, resulting in an intermediate residence time. Again, when 80% of the breakthrough capacity is reached at this particular flow rate, the flow rate is again lowered to allow a long residence time.
Variable loading is a familiar concept, but with modern protein A resin, the benefits are significantly improved.
With MabSelect SuRe LX, a productivity gain of close to 50% has been demonstrated with maintained capacity and purification performance. Variable loading has also been shown to improve the binding capacity of MabSelect SuRe LX resin.
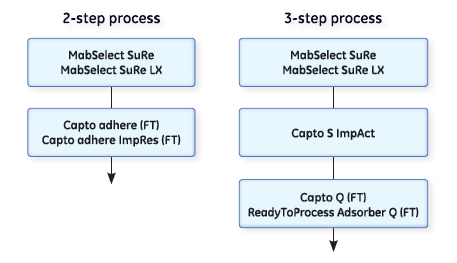
Polishing mAbs in a two-step process
The polishing steps following the capture step can be performed in either bind-elute (binding) or flow-through (nonbinding) mode. Our Capto adhere and Capto adhere ImpRes resins can both be used for polishing in a two-step process in flow-through mode. They are both based on the same multimodal anion exchange ligands and have similar ligand densities. This means they display high selectivity compared with traditional ion exchangers. They are both highly efficient in removing remaining impurities like aggregates, host cell protein (HCP), DNA, and viruses.
The choice between Capto adhere and Capto adhere ImpRes needs to be made on a case-by-case basis. They have different particle sizes. Capto adhere ImpRes has a smaller particle size of 40 μm compared with the 75 μm of Capto adhere resin. The smaller particle size enables higher resolution, while the larger particle size of Capto adhere gives the resin excellent pressure/flow properties. Selecting the most appropriate to get the results you want at high-throughput is essential.
Polishing mAbs in a three-step process
A three-step purification process, with two polishing steps based on one cation exchanger and one anion exchanger, is a classical way of purifying mAbs.
Cation exchangers are used for the removal of HCP, protein A, aggregates, and fragments. The cation exchange step is commonly followed by an anion exchange step (run in flow-through mode) for removal of remaining impurities such as DNA.
Chromatography resins and membranes suitable for polishing in a three-step purification process are Capto S ImpAct, Capto Q, and ReadyToProcess Adsorber Q.
Resins vs. membranes
In a chromatography membrane, the ligand which captures molecules is attached to a cellulose sheet rather than free-floating beads, as in a resin. The macroporous structure of the membrane surface, combined with its charge or hydrophobicity, make it suitable for capture and polishing of molecules at high flow rates.
To help you reduce process time and complexity, single-use, prepacked membranes like our ReadyToProcess Adsorber range offer advantages as they can be used straight away and don’t require cleaning, packing, testing, or validation. They also use less buffer than resins, reducing the need for floor space. Membranes can be a cost-efficient alternative for mAb polishing when the scale is smaller or in a low-frequency manufacturing scenario.
But traditional packed bed chromatography with resins like our Capto range can be more economical in large-scale operations. They also offer more versatility—valuable for challenging purifications.
Finding the sweet spot
With different resins offering a variety of advantages, it can make sense to evaluate the alternatives in order to achieve optimization. Screening, modelling, and small-scale column trials can help find the best combination for purity, volume and cost-efficiency.
See how the sweet spot for optimized purification was arrived at in three chromatography resin and membrane case studies.