Explore the workflow – hover over the products to learn more

Shorten your time to market with our flexible solutions
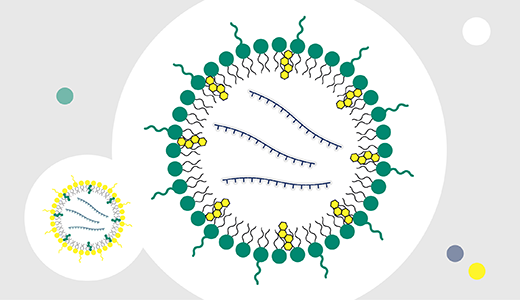
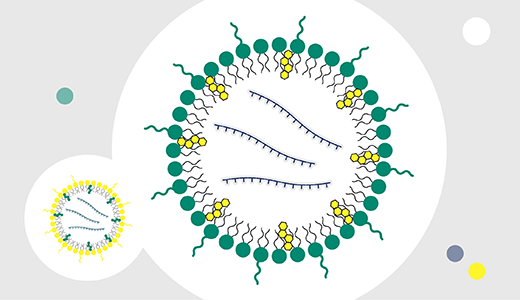
Figuring out how to accelerate mRNA therapeutics from development to commercialization can challenge even the best of us. But our teams are here to help support your full mRNA-LNP development and manufacturing process with our FlexFactory™ solutions.
Or we can extend your facilities with a prefabricated KUBio™ box. Providing rapid access to turnkey manufacturing of mRNA therapeutics, this solution can be on your site in as little as 10 months.
Explore FlexFactory™ solutionsDiscover more about KUBio™ modular facilities

End-to-end expertise, products and solutions
Discover how Cytiva can support your development and production needs across a variety of molecules and therapeutics.
Explore Cytiva solutions for mAbs, Cell therapies, Viral vectors