Cell therapies made a debut in the United States and Europe with the commercial launch of autologous chimeric antigen receptor (CAR) T cell therapies such as Kymriah™ and Yescarta™.
With more than 250 clinical trials worldwide studying CAR T cell therapies ongoing, additional cell therapies are on their way with potential indications broadening to include solid tumors from malignancies such as melanoma, pancreatic cancer, and glioblastoma. Headway is also being made for cell therapies derived from stem cells to potentially treat conditions like autoimmune diseases, Alzheimer’s, and Parkinson’s.
The success of cell therapies, however, hinges on not only proving that a product elicits the desired biological response but also overcoming the challenges of manufacturing and administering complex products to patients. To meet these supply chain challenges and deliver on the promise of cell therapies, companies need outstanding technologies and innovative strategies.
Because initial commercial cell therapies were rapidly developed with a focus on clinical outcomes, the time needed to build a robust, scalable manufacturing process was lacking. The commercial product came first and as a result, a host of manufacturing issues exists, including lack of automation, outdated analytics, and an immature supply chain.
Now that the industry is recognizing a need to scale cell therapy production to meet a great demand, there is an effort to industrialize processes and better incorporate supply chain considerations.
Cell therapy manufacturing is anything but conventional
In a conventional manufacturing operation, the process is confined to the manufacturing facility. But with cell therapies, this process extends far beyond, creating unique logistical challenges and responsibilities, as well as requiring a paradigm shift in thinking.
Cell therapy manufacturing begins with the collection of cells from the patient, which takes place in a clinical (or apheresis) facility and ends with the administration of the final drug product at the patient’s bedside.
Between initial collection of raw material and final administration of a product, dozens of hand-off points and processes take place. The complexity of all these moving pieces in a supply chain and the blending of the manufacturing and administration phases represent a new territory and are what differentiates the cell therapy field from more traditional pharmaceutical manufacturing.
A complete mapping of the entire manufacturing process is needed for a successful supply chain. A manufacturer must understand where the critical hand-off points are and understand the risks for each step.
Once a comprehensive map of all critical steps is in place, risk mitigation strategies must be incorporated, particularly with the introduction of automation. Because autologous cell therapies are personalized products with a batch size of one, manufacturers must also ensure that the chain of custody and identity is maintained throughout the entire manufacturing supply chain, no matter how complex.
Failure to document and control the provenance of the samples could have dire, even fatal, consequences for the patient. This diligent tracking also guarantees consistency, robustness, and quality control for the physicians and manufacturing team.
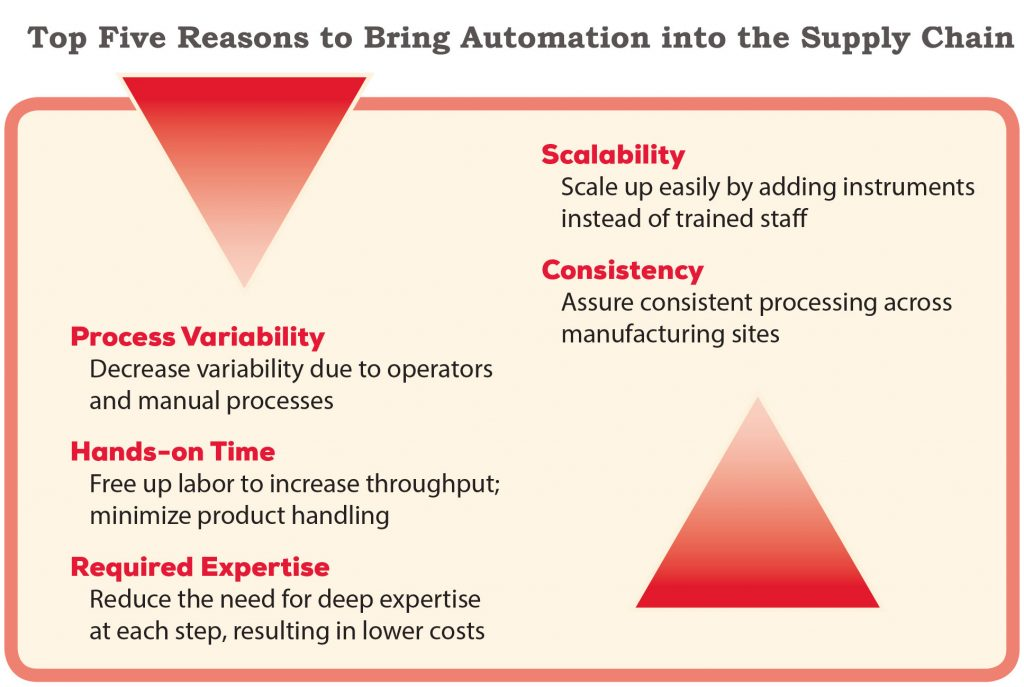
The individual nature of autologous cell therapies requires automation to decrease process variability and increase scalability.
In addition to the logistical challenges, cell therapy manufacturers must produce a consistently safe and effective product from highly variable starting material. Patients who typically provide the raw material (as is the case for autologous cell therapies) have different genetic backgrounds, stages of disease, and medical histories, all of which can introduce upstream complexity and variability into the production process.
In other words, the starting material is different every time, which is quite a change from the typical manufacturing scenario in which a well-defined cell line is used for each production run. “It comes down to the complexity of the systems that are involved: the fact that we are working with both a living starting material and a living product,” says Dr. Aaron Dulgar-Tulloch, Global Head of Research and Development, Director of Cell and Gene Therapy at Cytiva.
“That means that you have variability coming in your front end based on the donor and the condition of those cells,” continues Dr. Dulgar-Tulloch.
Adding automation to scale up
Most, if not all, cell therapies on the market are indicated for small patient populations. If these treatments are to become widely available across multiple indications, improvements in automation, process simplification, and supply chain management are needed to meet demand.
To scale up, manufacturing processes have to move from being open and manual to closed and automated, reducing contamination and risk, increasing product consistency and efficiency, and adding traceability throughout the process to ensure chain of custody.
With greater automation, operators can measure critical product attributes during critical process steps to better control for the variability of the starting material. Groups in both industry and academia are already adopting new technologies that can help them have more automated, closed, and scalable systems in order to remove risk and prepare for increases in future production.
Automation is particularly critical for producing single doses of autologous cell therapies because there is already so much risk of variability. That is why manufacturers can reap tremendous benefits from taking the time during process development to automate key steps and remove manual interaction.
Timing is another important aspect to automation—bringing it into the clinical development or research phase at the right time. If brought in too early, issues can arise further on; but brought in too late, efforts can be duplicated, repeating studies for regulatory authorities to prove that introducing an automated piece of equipment does not alter the quality of the product.
Contracting out the supply chain
Building a robust supply chain with the necessary equipment and protocols to meet demand can be difficult and time consuming for companies that are unfamiliar with the process.
An outside group—such as a contract manufacturing organization (CMO) or Cytiva—can help bridge the gap quickly for companies that have deep expertise in the biology of their product but lack experience in understanding how to move from a small, manual process to a scaled, automated production that can meet the needs of tens to hundreds of thousands of patients worldwide.
These organizations understand what kinds of experiments the client needs to carry out and how to run them to move an academic- or translational-scale process into something that can be scaled commercially. CMOs can also help to select the appropriate equipment. Outsourcing also helps manufacturers avoid investing in a large-scale system and facility in preparation for the future only to have their product delayed or not pass regulatory approval.
If a company does decide to outsource, they should strive to understand the process decisions so they can bring the process back in-house later if they choose to do so.
“You don’t want to just hand off development of your product without keeping yourself very engaged,” says Dr. Dulgar-Tulloch. “Otherwise, companies can quickly find themselves at the mercy of whatever CMO they have picked because all of the product manufacturing expertise for their product resides with the CMO.”
As for when to start thinking about the supply chain and outsourcing options, Dr. Dulgar-Tulloch’s advice is to start early on. If a company waits until the product is ready for commercialization, they will face “tremendous” delays and frustrations as they try to catch up or work within constraints they wish they could change.
Remove barriers to delivering your therapies with our cell and gene therapy solutions.