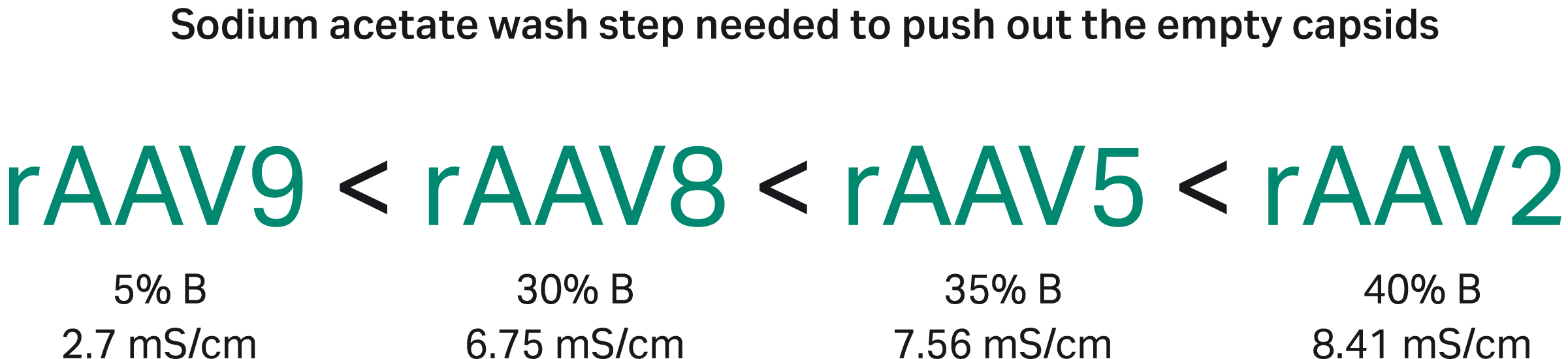Adeno-associated vector (AAV) is now established as the primary viral vector for gene therapy applications. Optimizing the polishing step of your AAV process for the separation of full capsids from empty capsids for each serotype is essential. This leads to improved viral genome (VG) recovery and a purity of full capsids.
We have developed a protocol for anion exchange with Capto™ Q chromatography resin, which allows near complete separation of full and empty capsids for multiple recombinant serotypes (rAAV2, rAAV5, rAAV8, and rAAV9). Previously, we have shown how Capto™ Q ImpRes chromatography resin can achieve VG recovery of approximately 60% to 70% and full capsid enrichment to between approximately 40% and 60%. To further improve full capsid recovery and purity, we will show how Capto™ Q resin with dextran surface extenders, magnesium chloride (MgCl2), and the elution salt type (especially for rAAV9) significantly enhance separation. Finally, we found that using Capto™ Q, a single protocol with sodium acetate in the elution buffer, and a step-elution approach greatly improved VG yields and full capsid purity to approximately 80% and above for all the serotypes tested.
Introduction
AAV is the main vector for gene therapy and there is a growing need for scalable, cost-efficient, and robust filtration and chromatography-based purification processes. One of the keys to a successful AAV workflow is achieving high overall yields of full capsids and efficient removal of empty capsid product impurities.
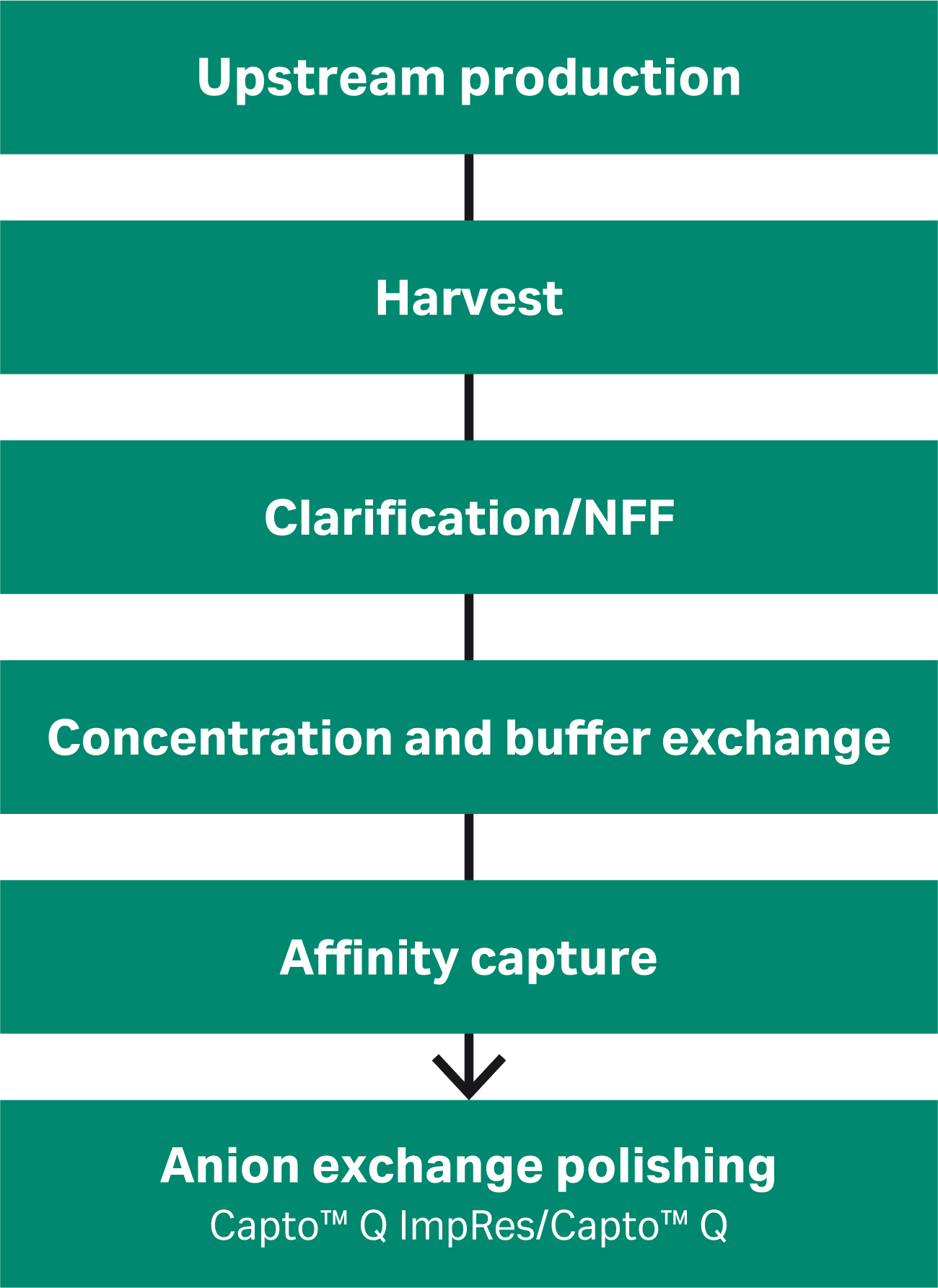
The start-to-finish AAV process for recombinant AAV5 (rAAV5) that we have developed can be seen in Figure 1, and each step is described in the following documents:
- Cell culture process development for AAV vector production in suspension cells
- Adeno-associated virus production in suspension HEK293 cells with single-use bioreactors
- Optimizing capture and polishing steps in an rAAV purification process
- Biacore™ surface plasmon resonance for titer analysis of adeno-associated virus
- Recombinant adeno-associated virus serotype 5 production process
Using the same the AAV process workflow, we produced different rAAV serotypes by triple plasmid transfection of HEK 293T cells in suspension. The rAAV was harvested by cell lysis and the lysate was treated with DNA nuclease, clarified, concentrated, and buffer exchanged before affinity capture. The affinity capture step does not discriminate between full and empty capsids on its own, so the polishing step is required to remove as many empty capsids as possible without negatively affecting VG yield.
Fig 1. An overview of the rAAV production process. How the anion exchange polishing was developed and improved for a range of rAAV serotypes is the focus of this article.
The separation of full and empty capsids can be achieved through ion exchange chromatography by utilizing the small difference in average pI between full capsids (pI 5.9) and empty capsids (pI 6.3). However, separation is often incomplete, and the peaks overlap. Therefore, optimization to maximize separation is critical.
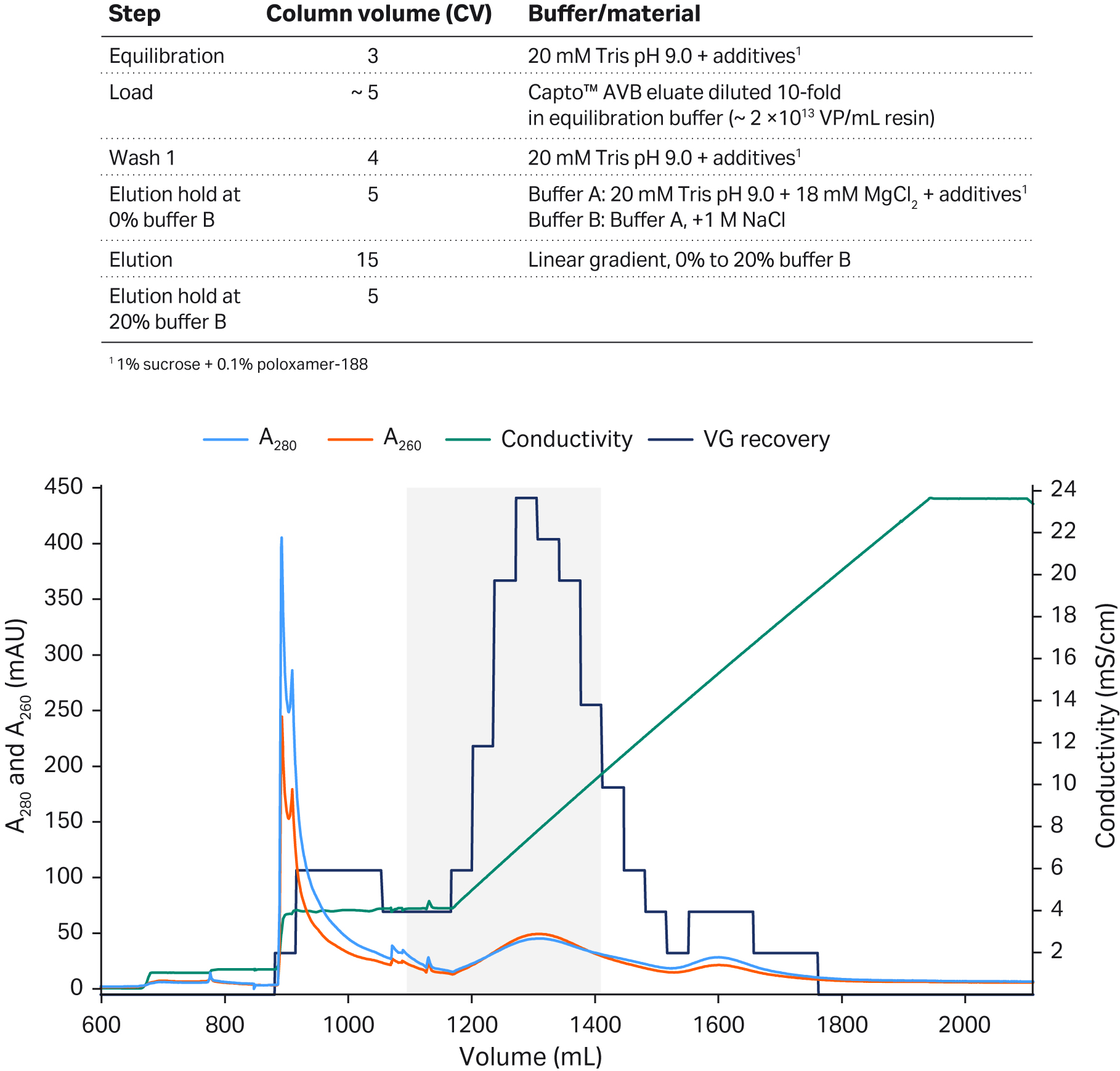
We have previously shown how we used Capto™ Q ImpRes anion exchange resin, which lacks dextran surface extenders for the ligand, for the separation of rAAV5 full capsids from empty capsids (Fig 2). In the polishing step, we developed an anion exchange protocol for rAAV5 where the empty capsids were first eluted with high MgCl2 concentration (18 mM), and the full capsids eluted when an NaCl gradient was applied (keeping the MgCl2 constant at 18 mM).
With the gradient elution used, we observed a recovery of 60% to 70% and 40% to 65% full capsids (as determined by the qPCR:ELISA ratio) depending on the pooling strategy (Fig 2). We found that there was a trade-off between VG recovery and % full capsids. The first peak contained mainly empty capsids while the second peak contained full capsids (confirmed by the qPCR:ELISA ratio). The UV260:280 ratio was just above 1.0 indicating that the sample in the second peak still contained empty capsids (1).
Fig 2. Separation of rAAV5 full and empty capsids using Capto™ Q ImpRes anion exchange (AIEX) chromatography resin. The optimized protocol included an 18 mM MgCl2 wash to elute the empty capsids followed by a NaCl linear gradient to elute the full capsids. The gray shaded area represents the pooled fractions containing the highest percentage of full capsids and sufficient viral genome recovery.
Based on these findings, we worked to develop an optimized polishing purification protocol for multiple rAAV serotypes — rAAV2, rAAV5, rAAV8, and rAAV9. In this article, we show you how the polishing step can be refined to yield more than 80% VG recovery and full capsid purity using:
- Anion exchange chromatography (AIEX) resin with dextran surface extenders (Capto™ Q resin),
- Optimization of MgCl2, and sodium acetate concentration in the elution buffer, and
- Step gradients for elution rather than linear gradients.
MgCl2 and dextran extenders enhance the separation of full and empty capsids
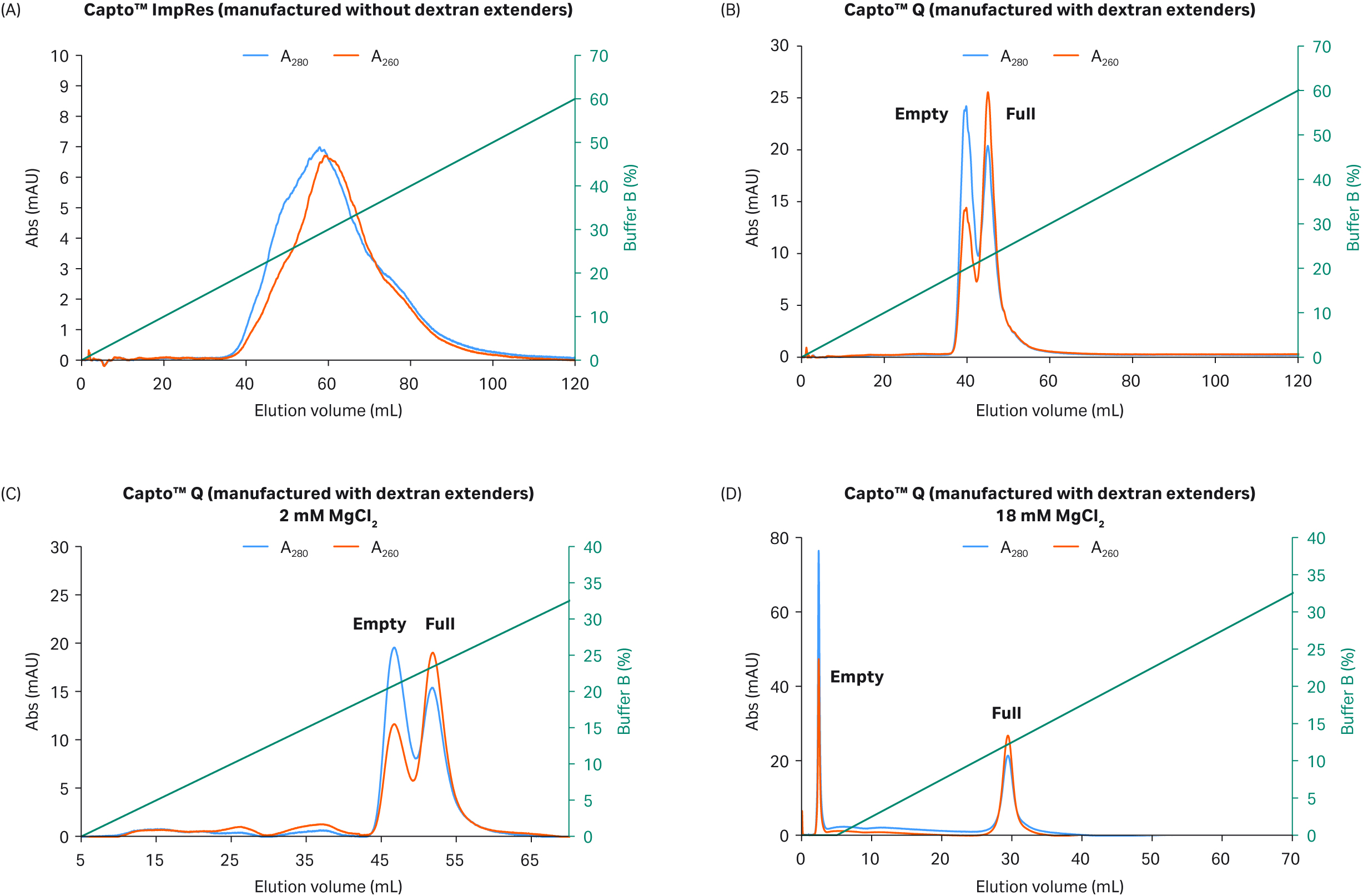
The lack of dextran extenders on the Capto™ Q ImpRes resin led to large peak overlap while the dextran extenders of Capto™ Q resin improved separation of rAAV8 and reduced overlapping peaks resulting in two visible peaks with a switch in the UV 260:280 ratio such that the first peak had a low ratio and the second a high ratio (Fig 3A and B). This effect was further enhanced when the dextran extenders of Capto™ Q resin were combined with a high (18 mM) concentration of constant MgCl2 in buffer A (Fig 3C and D).
Peaks on the chromatogram in Figure 3C, where 2 mM MgCl2 was used, were separated but with a clear peak overlap. While the buffer containing 18 mM MgCl2 resulted in elution of the empty capsids before the NaCl gradient was applied eluting the full capsids, resulting in well-separated peaks, as well as earlier elution in the linear NaCl gradient (Fig 3D). The mechanism of how MgCl2 enhances the separation is unclear, but it may be due to the differential binding of Mg2+ ions between full and empty capsids, which in turn affects binding to the anion exchange ligand.
Columns: Capto™ Q ImpRes or Capto™ Q resin packed in Tricorn™ 5/100 columns, 2 mL
Sample: ~ 1 × 1012 virus particles (VP) of rAAV8 (35% to 40% full capsids as determined by qPCR:ELISA)
Buffer A: 20 mM BTP pH 9.5, 2 mM MgCl2 + 1% sucrose and 0.1% poloxamer 188 additives
Buffer B: Buffer A + 400 mM NaCl
Gradient: Linear gradient elution with buffer B, 0 to 200 min
Flow rate: 1 mL/min
Fig 3. Separation of rAAV8 capsids on (A) Capto™ Q ImpRes (manufactured without dextran surface extenders) vs (B) Capto™ Q (manufactured with surface extenders) in the presence of 2 mM MgCl2. Separation performance of (C) Capto™ Q with 2 mM MgCl2 and (D) 18 mM MgCl2 is also shown. Elution was performed with an increasing NaCl linear gradient up to 400 mM.
Further improvement in separation through step gradients
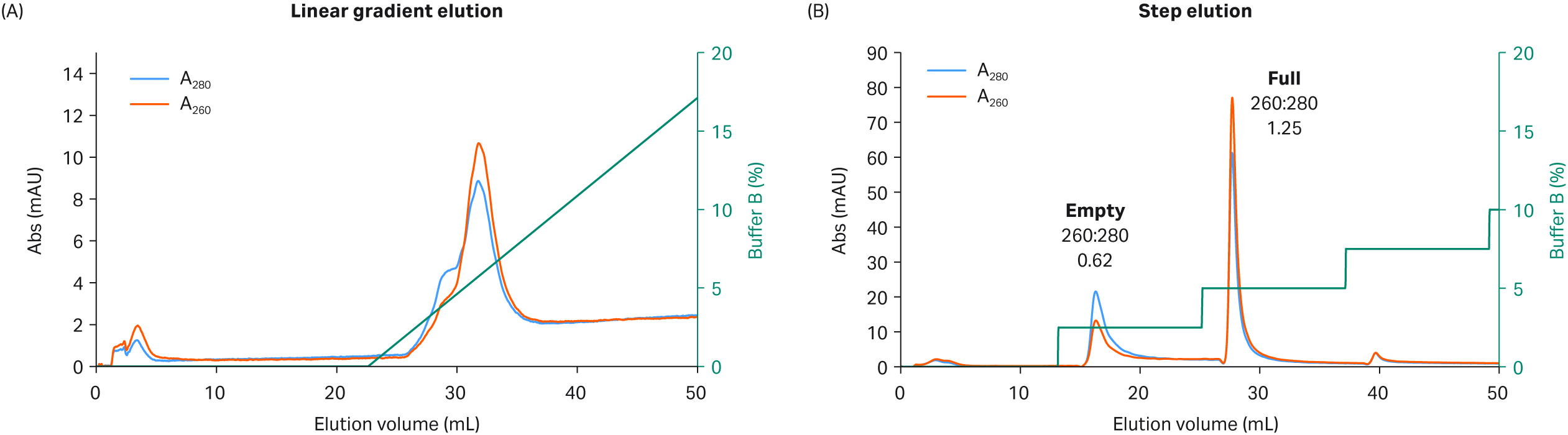
Gradient elution allows for small variations in experimental conditions — there is an advantage for flexibility in pooling fractions with the highest percent of full capsids when baseline separation of full/empty capsids is incomplete and peaks overlap. However, a step elution protocol improves separation performance (baseline separation is possible) and can simplify the process for large-scale GMP production. The full and empty capsid separation was clearly enhanced by the faster change (pulse) in conductivity during a step elution rather than the slow change occurring during a shallow linear gradient. The empty capsids seemed to be more efficiently and selectively released from the anion exchange ligand, leaving the full capsids still bound to the column before elution in a second step with higher salt concentration. This was demonstrated with the more challenging separation of full and empty rAAV5 capsids as compared to the easier rAAV8 serotype shown in Figure 4.
The good separation we achieved when we used an 18 mM MgCl2 wash was further improved with a step elution protocol rather than a linear NaCl gradient for the separation on Capto™ Q resin (Fig 4). The linear gradient elution resulted in the poor separation of rAAV5 with empty capsids eluted first as a shoulder of the second full capsid peak (Fig 4A).
Step elution, on the other hand, resulted in two well-separated peaks with high percentage of full capsids that can be seen by the increased UV 260:280 nm ratio (> 1.2) in the second peak of Figure 4B. The chromatogram illustrates the improvements that can be obtained by switching from gradient to step elution and reinforces the importance of the dextran extenders on the Capto™ Q resin.
Column: Capto™ Q resin in Tricorn™ 5/100 column, 2 mL
Sample: rAAV5
Sample load: 1.6 × 1012 VP (~ 60% full capsids as determined by qPCR:ELISA)
Buffer A: 20 mM BTP pH 9.5 + 18 mM MgCl2 + additives (1% sucrose + 0.1% poloxamer 188)
Buffer B: Buffer A + 400 mM NaCl
Flow velocity: 2 mL/min
Gradient: Gradient elution with 40 CV of buffer B. Step elution, buffer B (6 CV): 2.5%, 5%, 7.5% (10 mM NaCl increments) were used
System: ÄKTA pure™ 25
Detection: 260 nm and 280 nm
Fig 4. Separation of rAAV5 full and empty capsids using Capto™ Q and (A) a linear gradient elution or (B) a step elution protocol with high constant concentration of MgCl2 (18 mM) and NaCl as elution salt.
Developing a single polishing protocol for multiple rAAV serotypes
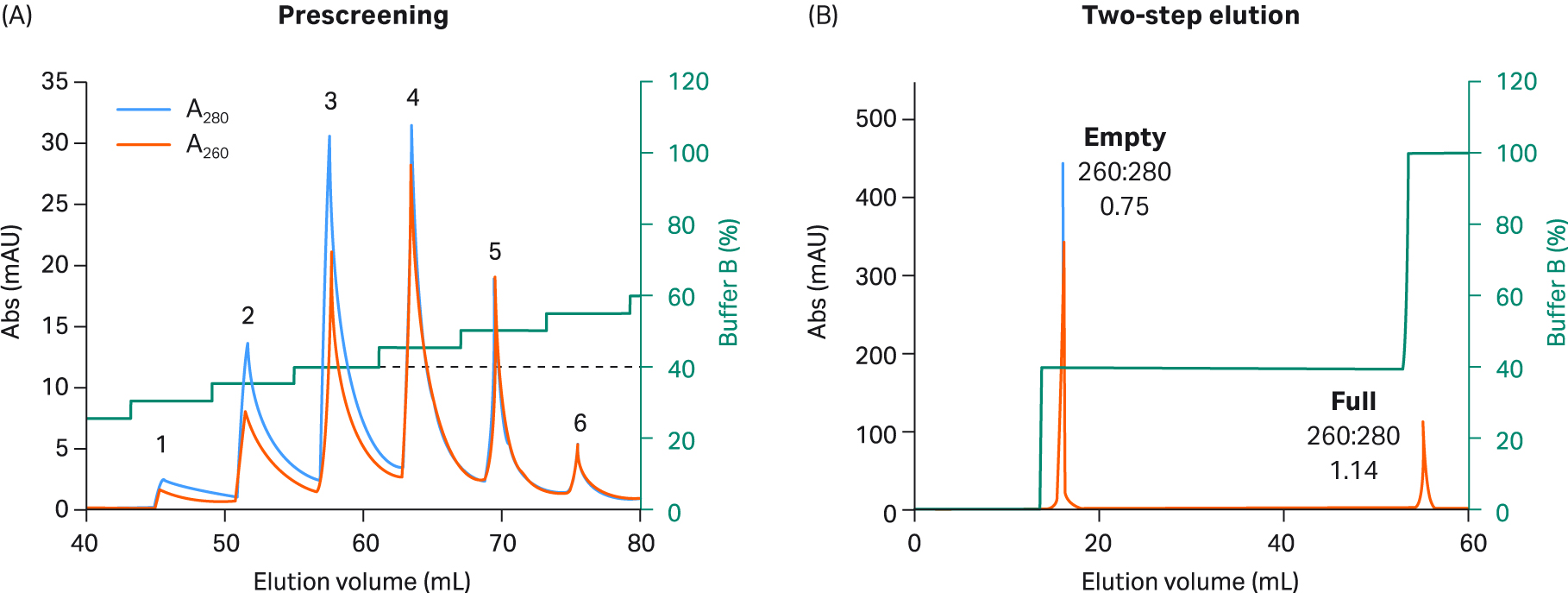
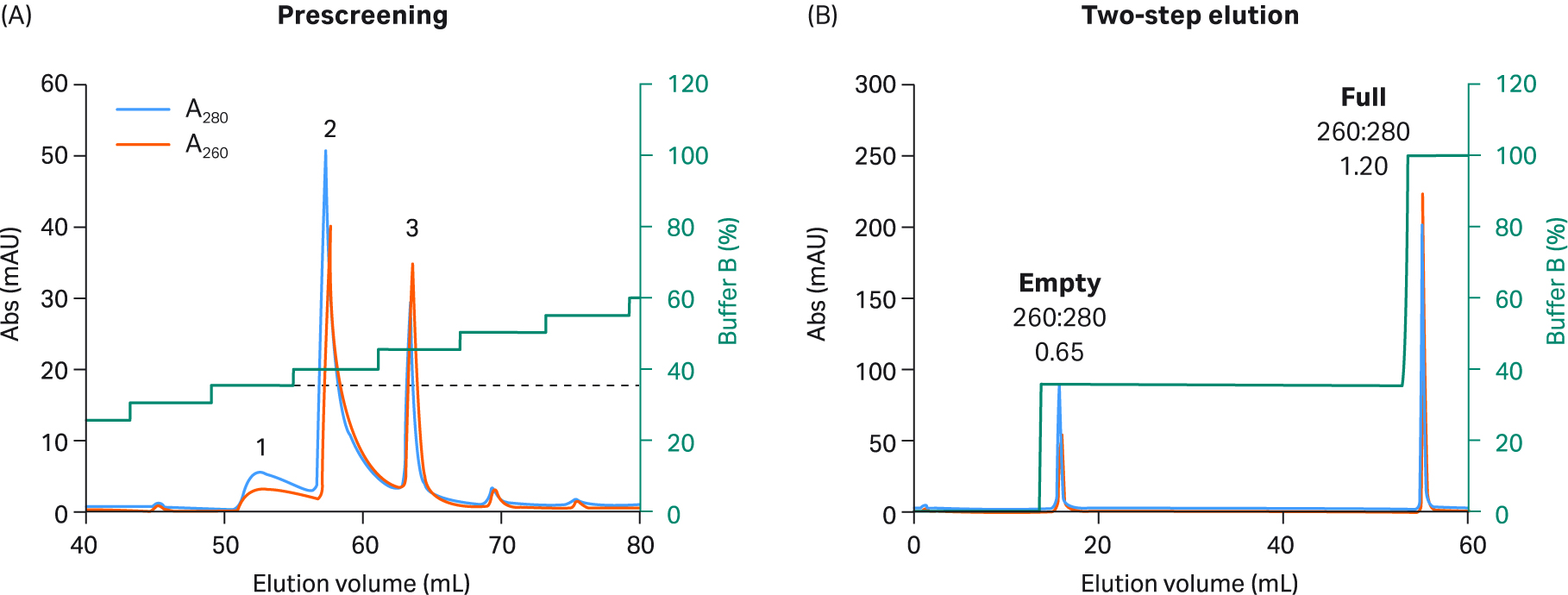
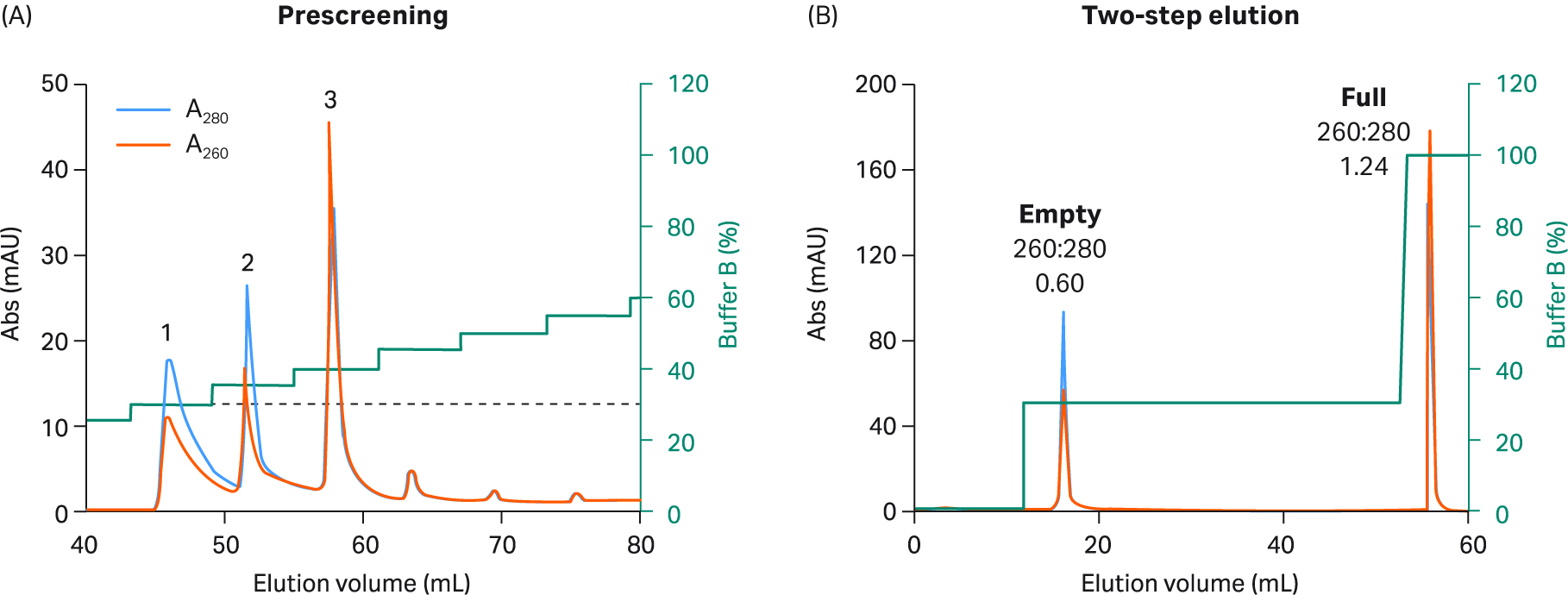
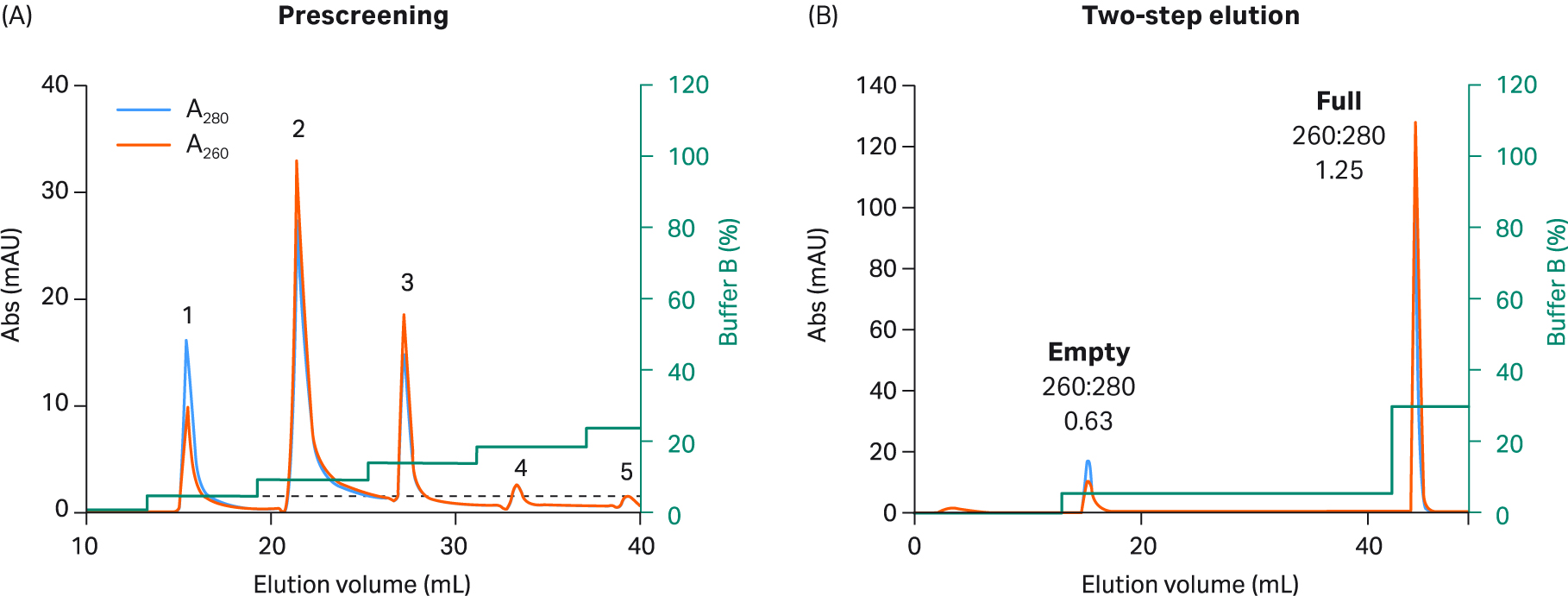
We explored full and empty capsid separation for recombinant AAV2, AAV5, AAV8, and AAV9 serotypes using AIEX with Capto™ Q and a two-step elution. We were able to develop an optimized step elution protocol that worked well for all four serotypes. It was also designed to be compatible with AAV9 which binds weakly to the anion exchange resin. We used 20 mM bis-Tris propane (BTP), pH 9.0 containing constant 2 mM MgCl2 without additives. We also replaced NaCl as an elution salt with sodium acetate, which is a milder, more kosmotropic (order-inducing) salt. The elution conditions required to elute the empty capsids were identified in a prescreening procedure using incremental 5% elution buffer (buffer B) steps of 3 CV each (Fig 5 to 8, panel A).
These small incremental elution steps result in several peaks eluting, the first one or two containing empty capsids, followed by peaks in the middle containing a mix of full and empty capsids, and the last ones containing the full capsids. This can be seen by the changes in the UV 260:280 ratios for the different peaks going from lower to higher. This buffer system has a low tendency to give background UV signals as measured by blank runs with buffer alone without rAAV sample (data not shown).
Optimizing the two-step elution protocol
Based on the results of our prescreening, we used the percentage of buffer B where empty capsid elution was initiated and before elution of full capsids started (leakage of full into the empty capsid peak) for the first step. This can be seen as the dotted line in panel A in Figures 5 to 8. We also extended step 1 to 20 CV to maximize the empty capsid removal. This was followed by a second, shorter (5 CV) step for elution of full capsids using 100% buffer B or lower depending on the elution concentration for the full capsids in the prescreening run.
The results from the final two-step elutions are shown in Figure 5 to 8, panel B. rAAV2 had a very low percentage of full capsids in the start material and the second full peak was smaller with a slightly lower UV260:280 ratio than for the other serotypes (Table 1). The enrichment factor for rAAV2 was very high as seen from the change in UV ratio indicating a change from 7% to > 70% full capsids (estimated based on UV260:280 of 1.14). The UV260:280 ratio in the peaks gives a good indication of the degree of purification in the chromatograms. We also confirmed the general observation through qPCR (VG) and ELISA (viral particle, VP) ratio analysis, and calculated the VG recovery (Table 1).
The qPCR:ELISA ratio can give variable, and therefore ambiguous results; however, the combination of UV260:280 ratio and qPCR:ELISA results in peaks 1 and 2 taken together give a strong indication of excellent recovery of full capsids and a high percentage of full capsids in peak 2. We recommend combining analysis methods to maximize accuracy by adding orthogonal methods such as analytical ultracentrifugation (AUC), analytical ion exchange (IEX), or transmission electron microscopy (cryoTEM) whenever possible.
The capacity of both Capto™ Q ImpRes and Capto™ Q resins was estimated to be at least 1 to 3 × 1013 VP/mL resin. The UV260:280 ratio of 0.6 to 0.7 indicated mainly empty capsids while a ratio > 1.2 indicated mainly pure full capsids. The extinction coefficients for specific serotypes can be considered and used to calculate the rAAV concentration for higher accuracy (1). The UV values could be affected by insert size and the presence of small amounts of HCP or DNA. We recommend the use of a UV detector with a 10 mm path length for sufficient detection sensitivity providing higher UV260:280 accuracy.
Prescreening protocol
Column: Capto™ Q resin packed in Tricorn™ 5/100, 2 mL column
Sample load: ~ 1 × 1012 VP/mL resin
Buffer A: 20 mM BTP, pH 9.0, 2 mM MgCl2
Buffer B: 20 mM BTP, pH 9.0, 2 mM MgCl2, 250 mM Na acetate
Equilibration: Buffer A, 5 CV
Wash: Buffer A, 5 CV
Gradient: Step elution, 5% increments, 3 CV each
Flow rate: 2 mL/min
System: ÄKTA pure™ 25
Two-step protocol
Column: Capto™ Q Tricorn™ 5/100, 2 mL column
Sample load: (1 × 1012 VP/mL resin)
Buffer A: 20 mM BTP pH 9.0, 2 mM MgCl2
Buffer B: 20 mM BTP pH 9.0, 2 mM MgCl2, 250 mM Na acetate
Equilibration: Buffer A, 5 CV
Wash: Buffer A, 5 CV
Gradient: Two-step elution according to the following for each serotype:
rAAV2: Step 1 40% buffer B, 20 CV
Step 2 100% buffer B, 5 CV
rAAV5: Step 1 35% buffer B, 20 CV
Step 2 100% buffer B, 5 CV
rAAV8: Step 1 30% buffer B, 20 CV
Step 2 100% buffer B, 5 CV
rAAV9: Step 1 5% buffer B, 20 CV
Step 2 30% buffer B, 5 CV
Flow rate: 2 mL/min
System: ÄKTA pure™ 25
Fig 5. (A) Prescreening using 5% incremental steps with buffer B and Capto™ Q resin to select two step elution conditions for rAAV2. rAAV2 empty capsids were eluted in the first three peaks. A mixture of full and empty capsids was eluted in peak four. Finally, full capsids were eluted in the fifth and sixth peaks. The percentage of buffer B for step one was selected (dotted line) based on the concentration needed for the first empty capsid peak to elute during prescreening without elution leakage of full capsids. (B) For the final rAAV2 2 step protocol, 20 CV of 40% buffer B was used in step one (corresponding to prescreen peak 3), followed by 5 CV of 100% buffer B in step two.
Fig 6. (A) Prescreening using 5% incremental steps with buffer B and Capto™ Q resin to select two-step elution conditions for rAAV5. During prescreening, rAAV5 was divided into fewer peaks and needed a lower percentage of buffer B to elute the first empty peak. (B) For the final rAAV5 2 step protocol, 20 CV of 35% buffer B was used in step one (corresponding to peak 1 in prescreening), followed by 5 CV of 100% buffer B in step two.
Fig 7. (A) Prescreening using 5% incremental steps with buffer B and Capto™ Q resin to select two-step elution conditions forrAAV8. Like rAAV5, rAAV8 was also divided into fewer peaks and needed an even lower percentage of buffer B to elute the first empty peak. (B) For the final rAAV8 2 step protocol, 20 CV of 30% buffer B was used in step one (corresponding to peak 1 in prescreening), followed by 5 CV of 100% buffer B in step two.
Fig 8. (A) Prescreening using 5% incremental steps with buffer B and Capto™ Q resin to select two-step elution conditions for rAAV9. The interaction of rAAV9 is different than the other serotypes. As a result, the required amount of buffer in step one was much lower. (B) For the final rAAV9 2 step protocol, 20 CV of 5% buffer B was used in step one, followed by 5 CV of 30% buffer B in step two. You could also use 100% buffer B in step two, but this is not required due to the weak binding of rAAV9.
The binding strength relationships between the serotypes were clearly demonstrated by the amount of buffer B that was needed to elute the empty capsids (Fig 9).
The conductivity numbers shown in Figure 9 are for the buffer system and pH used in this protocol.
Using a different buffer with the same conductivity may yield different results. The exact conductivity required to elute the empty can also be affected by the specific capsid structure and insert size used. The relative binding strength to the anion exchange for capsids with a GFP insert was: rAAV9 < rAAV8 < rAAV5 < rAAV2 as seen by the increasing percentage of buffer B needed for elution (Fig 9).
We recommend ensuring a conductivity between 1 to 3 mS/cm in the loaded material to avoid the risk of poor separation or all material flowing through without binding. This can be achieved by neutralizing and diluting the affinity capture eluate before the polishing step. For rAAV9 that binds weaker to the anion exchange, we recommend that the load conductivity is below 2 mS/cm.
Fig 9. The relative binding strength to the anion exchange resin was different for the different rAAV serotypes as seen by the increasing the percentage of buffer B needed for elution.
Table 1. Summary of separation results for full and empty capsids and VG recovery from four rAAV serotypes on Capto™ Q chromatography resin
| Serotype | Start sample | Peak 1 (empty capsids)1 | Peak 2 (full capsids)1 | ||||
| qPCR:ELISA (full capsids, %) | UV 260:280 (peak area) | VG recovery2 (%) | qPCR:ELISA (full capsids, %) | UV 260:280 (peak area) | VG recovery2 (%) | qPCR:ELISA (full capsids, %) | |
| rAAV2 | 7.0 to 10.0 | 0.75 | N/A | N/A | 1.14 | N/A | N/A |
| rAAV5 | 47.0 | 0.65 | 7.0 | 5.0 | 1.20 | 80 | 100 |
| rAAV8 | 11.0 to 35.0 | 0.60 | 3.0 | 1.0 | 1.24 | 80 | 95 |
| rAAV9 | 40.0 | 0.63 | 0.3 | 1.0 | 1.25 | 91 | 100 |
1Mass balance of UV absorbance based on total UV signal was 70% to 100%.
2VG = viral genomes.
Conclusions
- Capto™ Q ImpRes allows full capsid enrichment to approximately 50% but Capto™ Q with dextran surface extenders enhances the separation, improving VG yields and purity to ~ 80% or above.
- MgCl2 is critical in the wash and elution steps — Mg2+ ions seem to differentially bind to full and empty capsids and enhance the separation although the exact mechanism of the interaction is unknown.
- Step elution separated significantly better than gradient elution.
- Prescreening is recommended to identify optimal step elution conditions.
- Optimal separation was provided with our one-buffer solution for multiple rAAV serotypes — rAAV2, rAAV5, rAAV8, and rAAV9 (and likely also other serotypes) — using 20 mM BTP, pH 9.0, 2 mM MgCl2 with 250 mM sodium acetate as elution salt.
- Conductivity < 3 mS/cm or < 2 mS/cm (rAAV9) in the sample before loading is critical.
Get tips to improve your viral vector process in this article (5 min read)
References
- Dickerson R., Argento C., Pieracci J., Bakhshayeshi M. Separating empty and full recombinant adeno-associated virus particles using isocratic anion exchange chromatography. BioTechnology J. 2020;16(1).
- Venkatakrishnan B, Yarbrough J, Domsic J, Bennett A, Bothner B, Kozyreva OG, Samulski RJ, Muzyczka N, McKenna R, Agbandje-McKenna M. Structure and dynamics of adeno-associated virus serotype 1 VP1-unique N-terminal domain and its role in capsid trafficking. J Virol. 2013 May;87(9):4974–4984. Epub 2013 Feb 20. PMID: 23427155; PMCID: PMC3624325.
Materials and methods
rAAV production
Human embryonic kidney-293T (HEK293T) CRL-3216 ATCC cells adapted to suspension culture were grown in HyClone™ HyCell™ TransFx-H medium using Xcellerex™ XDR-10 and ReadyToProcess WAVE™ 25 bioreactors. An optimized triple plasmid (Rep/cap: helper: transgene [green fluorescent protein] GFP) transfection protocol was used to produce rAAV2-GFP, rAAV5-GFP, rAAV8-GFP, and rAAV9 GFP (1, 2).
Harvest and clarification
At the time of harvest, we lysed the cells with 0.5% Tween™ 20 + 150 mM NaCl and treated them with 40 U/mL Denarase™ nuclease (c-LEcta) + 1 mM MgCl2 for 4 h in the bioreactor. To avoid possible spontaneous precipitation, we treated the feed with pH 4.0 (by addition of 1 citric acid) for 30 min before neutralizing to pH 7.0 (by addition of 1 M Tris base) and the resulting precipitate was allowed to sediment and discarded. The cell lysate was clarified by normal flow filtration (NFF) using a combination of 5 µm and 2 µm GF as well as 0.6/0.2 μm HC ULTA™ filters, each at approximately 500 cm2/L harvest feed (see how to build a scalable rAAV production process in this application note).
Tangential flow filtration (TFF)
The clarified feed was concentrated 10-fold by TFF on a ReadyToProcess™ hollow-fiber filter (UFP-300-C-4x2MA) using an ÄKTA flux™ 6 tangential flow filtration system. The filter area of this filter cartridge was 1400 cm2 with a NMWC of 300 000. The membrane flow rate was approximately 140 cm2/L clarified feed with a shear rate of 7000 to 9000 s-1 and transmembrane pressure (TMP) of 0.09 MPa (0.9 bar, 13.1 psi). After concentration, a five-fold volume buffer exchange was performed to 20 mM Tris pH 7.8, 200 mM NaCl.
Affinity capture
We used prepacked columns for affinity capture of recombinant rAAV2, rAAV5, rAAV8, and rAAV9. Capto™ AVB HiTrap™ 5 mL chromatography column was used for rAAV2 and rAAV5, POROS™ Capture Select™ columns (Thermo Fisher Scientific) for rAAV8 and rAAV9. Columns were operated on an ÄKTA pure™ 25 chromatography system and the flow velocity of 150 cm/h was equivalent to 1 min residence time for all steps using the supplier-recommended methods. After the low pH elution, the sample was neutralized by the addition of a 10% volume of 1 M Tris-HCl, pH 9.0.
The sample load was approximately 1 to 3 × 1014 VP/mL of resin.
Polishing
The neutralized affinity capture eluate was diluted 10-fold in an equilibration buffer without MgCl2 (A buffer) to a conductivity of between 1 to 3 mS/cm. A Capto™ Q ImpRes HiScale™ column with a column volume of 51 mL and a bed height of 10 cm or a Capto™ Q Tricorn™ 5/100, 2 mL column was used. Columns were operated on an ÄKTA pure™ 25 system using the methods described in the figures and the flow velocity of 250 cm/h was equivalent to 2.4 min residence time (Capto™ Q ImpRes) or 150 cm/h equivalent to 1 min residence time (Capto™ Q) for all steps. The sample load was approximately 1 × 1013 VP/mL of resin.
Analytics
Total rAAV particle VP titer was determined using AAV2 or AAV5 ELISA (Progen). Viral genome (VG) titer was analyzed using qPCR targeting the cytomegalovirus (CMV) sequence. The ratio of qPCR:ELISA was used to estimate the percentage of full capsids. The ratio of UV260:280 nm was used to indicate the percentage of viral capsids in the chromatograms.
CY30251-10Oct22-AN