We developed a recombinant adeno-associated virus serotype 5 (rAAV5) bioreactor production process — ranging from the cryovial to the purified, sterile filtered bulk product. The process is closed and based on serum-free culture in HyCell™ TransFx-H medium. No animal derived components are used, and the complete process is compatible with GMP requirements.
Here, we describe how we expanded HEK293/HEK293T suspension cells from small cell culture (20 mL) in shake flasks up to a 10 L culture in a benchtop Xcellerex™ XDR-10 single-use bioreactor system. AAV was produced at high titers upon transient plasmid transfection in the bioreactor. We also produced rAAV5 in ReadyToProcess WAVE™ 25 bioreactor system, up to 20 L. Furthermore, we present data that the process is suitable also for other serotypes such as AAV2, AAV8, and AAV9 in addition to AAV5.
Introduction
Viral vectors are increasingly used for gene transfer to specific tissue or to induce cell type modifications. Several viruses have been investigated for their use in cell and gene therapy with recombinant adeno-associated virus (rAAV) as the most widely used vector for gene therapies.
One significant challenge in the gene therapy manufacturing industry is to establish large-scale production in accordance with current good manufacturing practices (GMP). The regulatory requirements for viral vectors are still evolving and are expected to increase.
Another trend in the field of recombinant AAV (rAAV) production is a move away from using adherent HEK293 cells and a move toward scalable closed bioreactor technologies based on suspension HEK293 cells. These are used to produce high-titer rAAV with transient transfection technology.
In this work, the rAAV5 serotype was selected to showcase rAAV production. HEK293T cells were adapted in HyCell™ TransFx-H to suspension and serum free conditions as previously described and transfected to produce rAAV5. The adapted HEK293T cells were expanded and scaled up in Xcellerex™ XDR-10 and ReadyToProcess WAVE™ 25 bioreactors for production of rAAV5. Single-use bioreactors are preferred for viral vector production due to several reasons. For example, the risks for cross contamination are highly reduced and production capacity could increase due to shorter change over procedures between batches. Also, flexibility and scalability in the production plant are increased with single-use bioreactors.
HEK293T cells were transfected by a triple-plasmid expression using polyethylenimine (PEI) as the DNA transporter. For successful generation of functional viral particles, cells need to be transfected with all three plasmids at suitable ratios. The three plasmids that we used in this study are:
(1) pAAV-GFP control containing a GFP reporter gene
(2) pHelper plasmid containing the adenovirus genes E2A, E4, and VA RNA
(3) pAAV-RC5 vector plasmid containing the replication proteins and AAV5-specific capsid proteins
The optimization of the transfection conditions using a Design of Experiments (DoE) methodology has been described previously.
Our goal with the study was to achieve 1010 viral genomes (VG)/mL (1013/L), and 1011 of viral particles (VP)/ mL (1014/L) in crude harvest material. These criteria are based on levels described in the literature. For percentage of full capsids, we aimed for at least 10% full capsids in the harvest material to allow efficient separation of full and empty capsids during the purification process. The percentage full capsids were calculated as a ratio between VG/mL and VP/mL.
Detailed Materials and methods are found at the end of the document.
Verification runs in bioreactors at the 10 L scale
In our rAAV5 production process, we started from a small cell culture volume in shake flasks and expanded cells in a single-use bioreactor. During the process development stage, we developed a transfection method specifically for larger volume production of rAAV5.
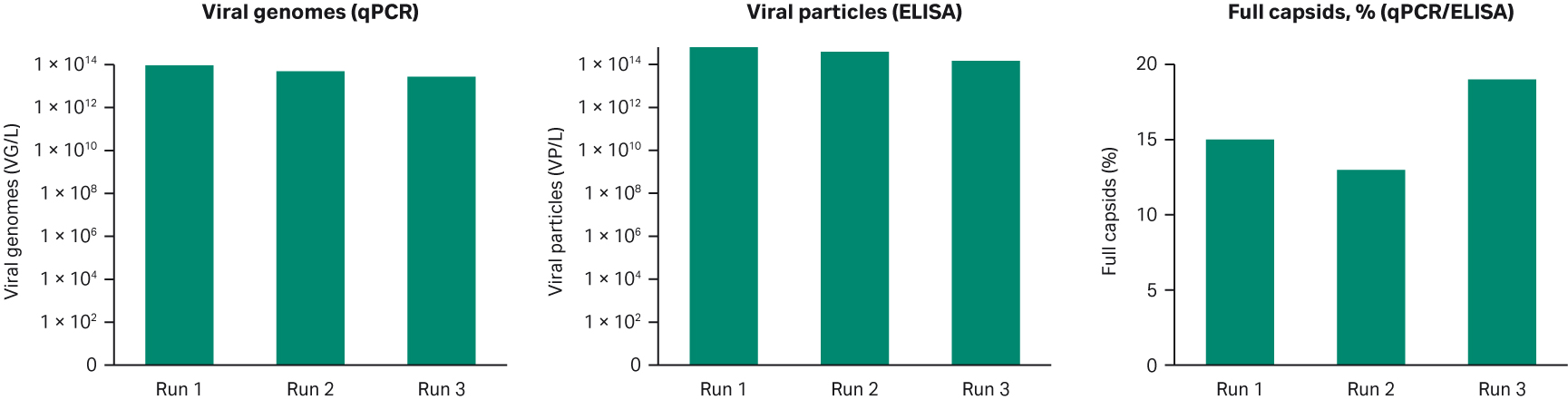
As mentioned above we needed at least 1010 VG/mL (1013/L), 1011 VP/mL (1014/L) with more than 10% full capsids in the harvest material. The percentage of full capsids was calculated as a ratio between VG/mL (analyzed with qPCR) and VP/mL (analyzed with ELISA).
We performed verification runs at 10 L scale in XDR-10 and WAVE™ 25 bioreactors before the final process to ensure that the transfection protocol met our target for rAAV5 productivity.
Results from the verification on production batches are shown in Figure 1. All our expected criteria were met with these initial batches.
Fig 1. Verification batches for rAAV5 at 10 L scale using Xcellerex™ XDR-10 (left) and WAVE™ 25 bioreactor (right) confirmed that criteria for rAAV5 production could be achieved with the developed transfection protocol.
Production in XDR-10 bioreactor
The production of rAAV5 in XDR-10 bioreactor is summarized as follows:
First, cells were expanded to generate an inoculum for the XDR-10 bioreactor. HEK293T cells were expanded in shaker flasks to produce an inoculation cell concentration of 0.4 × 106 cells/mL in a 5.5 L starting volume.
The XDR-10 bioreactor was set up for inoculation and growth of HEK293T suspension cells to a density of approx. 2 × 106 cells/mL. Cells were diluted by addition of 4 L of complete HyCell™ TransFx-H culture medium, which gives a working volume of 9.5 L.
Transfection was performed according to our optimized protocol and the transfection complex solution was added to the bioreactor with a final bioreactor volume of 10 L and a cell concentration of 1 × 106 cells/mL.
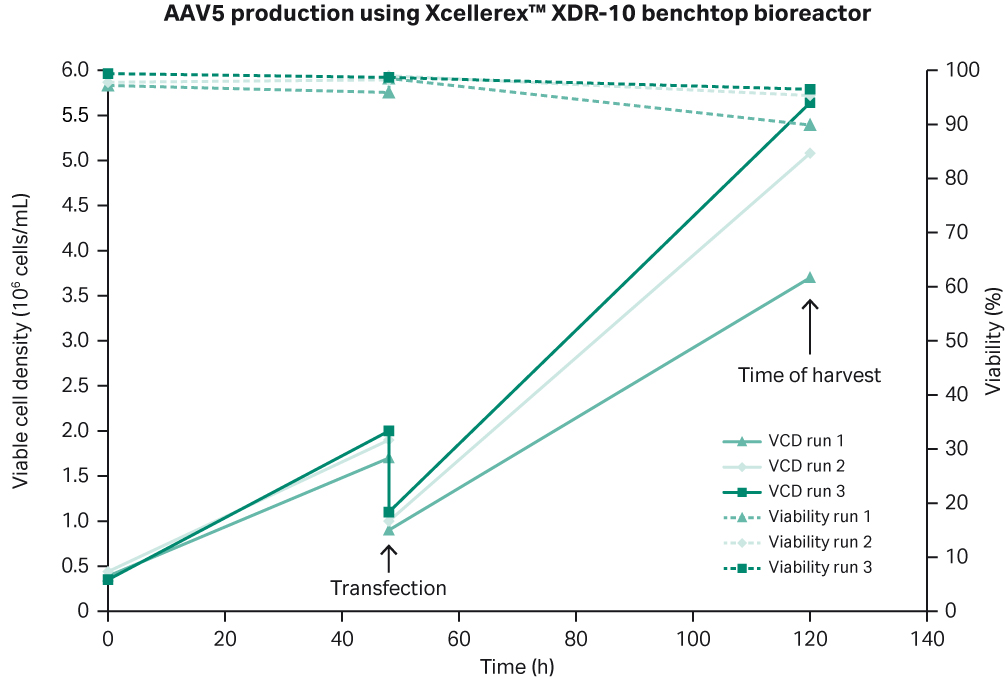
The results from the three production batches in XDR-10 bioreactor are shown in Figure 2. Different spargers were used for run 1 compared to runs 2 and 3, which may have resulted in better oxygenation in runs 2 and 3. This in turn could account for the improved viable cell density (VCD) and viability.
Fig 2. Summary of three rAAV5 production batches in Xcellerex™ XDR-10 bioreactor.
Transfection efficiency evaluation
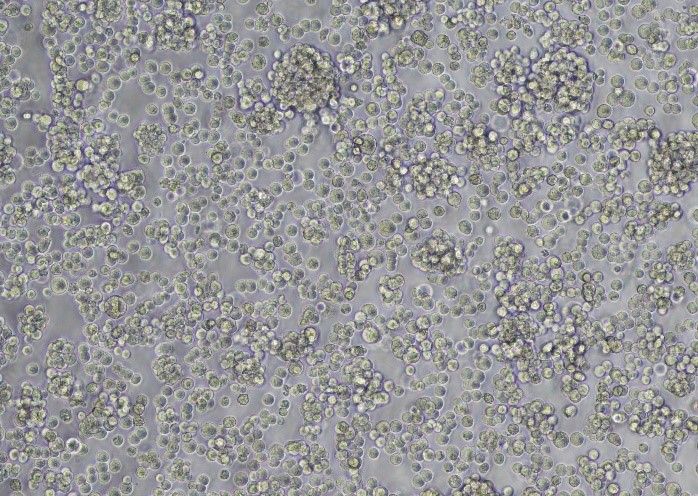
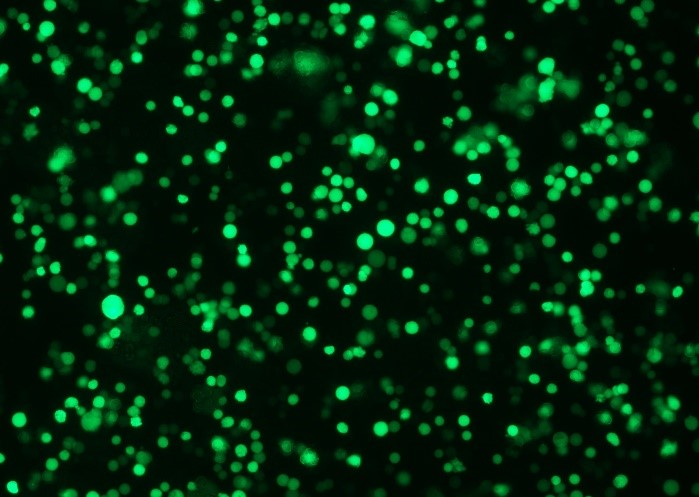
To confirm transfection efficiency in the XDR-10 bioreactor, we performed brightfield and fluorescent microscopic evaluation of cells in addition to flow cytometry analysis. Figure 3 shows GFP-positive fluorescing cells in diluted sample of rAAV5 at harvest.
(A)
(B)
Fig 3. (A) Bright field and (B) fluorescent microscope images from diluted sample of rAAV5 at harvest from the XDR-10 bioreactor.
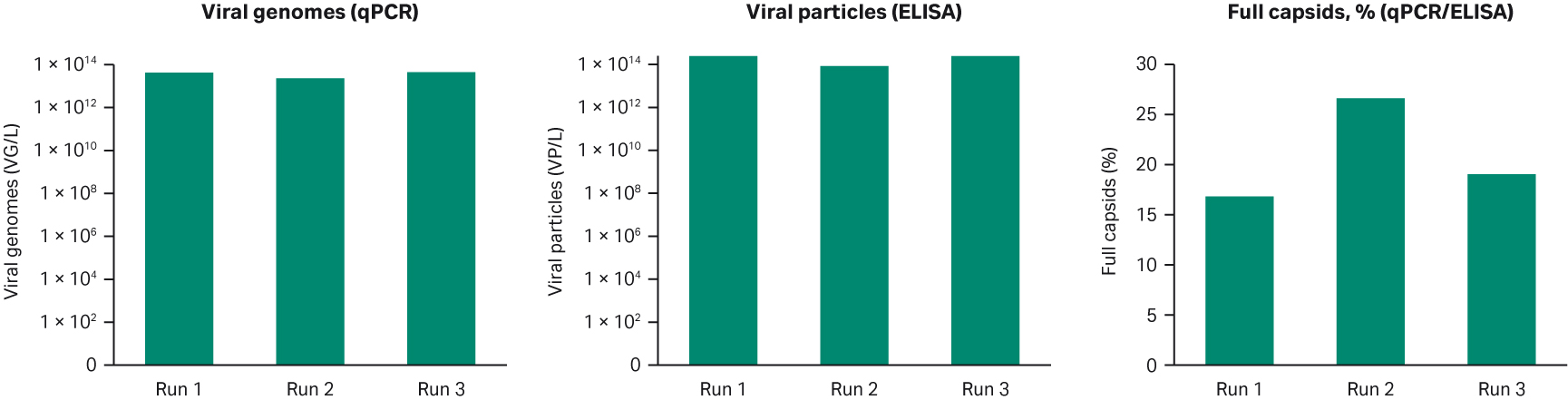
The production batches gave a similar viral titer with approximately 1014 VP/L and all three production batches successfully yielded > 10% full capsids (Fig 4). The consistency between all three batches indicates that the process design is robust and suitable for manufacturing purposes.
Fig 4. Summary of the three production batches in XDR-10 bioreactors. All criteria were met with respect to viral genomes, viral particles, and percentage of full viral capsids for rAAV5 production.
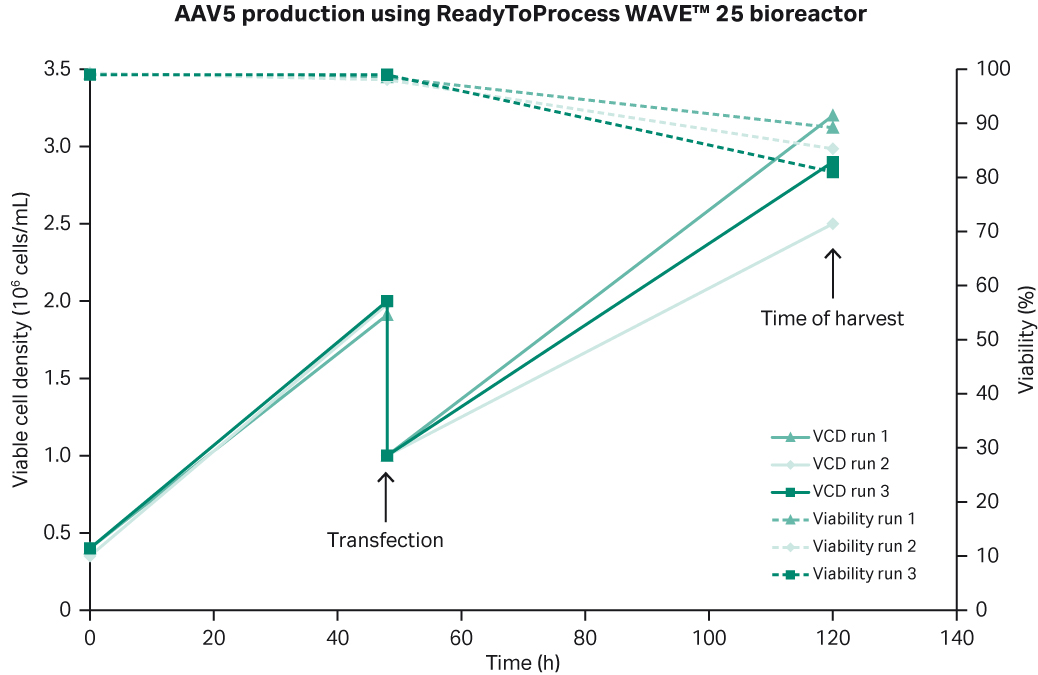
Production in WAVE™ 25 bioreactor
We wanted to test if a WAVE™ type of single-use bioreactor (rocking motion) could be used as an alternative to the XDR (stirred-tank design). Thus, we needed to verify the performance of the AAV transient transfection protocol and production in the WAVE™ 25 bioreactor.
First, HEK293T cells were grown and expanded in shaker flasks to produce an inoculation cell volume of 0.4 × 106 cells/mL in a 10 L starting volume.
The WAVE™ 25 bioreactor was set up for inoculation and growth of HEK293T suspension cells to approx. 2 × 106 cells/mL.
Cells were diluted by addition of 9 L fresh supplemented HyCell™ Trans-Fx-H culture medium, which gave a working volume of 19 L.
Transfection was performed according to our optimized protocol and the transfection complex solution was added to the bioreactor to a final volume of 20 L and a cell concentration of 1 × 106 cells/mL.
In the three production batches in WAVE™ 25 bioreactor, we observed reproducible results with respect to cell growth and viability (Fig 5).
Fig 5. Summary of three rAAV5 production batches in WAVE™ 25 bioreactor.
For the rAAV5 production batches in WAVE™ 25 bioreactor, we also looked at scalability — in run 1 and 2 we produced the larger 20 L cell culture volume while in run 3, it was 10 L. The criteria we had set for viral genome, viral particles, and percentage of full capsids for the different production batches were all met at the larger scale (Fig 6).
Fig 6. Summary of the three production batches in WAVE™ 25 bioreactor. All criteria were met with respect to viral genomes, viral particles, and percentage of full viral capsids for rAAV5 production.
Thus, we could confirm that the WAVE™ 25 is also suitable for AAV production with similar productivity as the XDR-10 bioreactor. While good results were observed for both bioreactors, the advantage of the process in a Xcellerex™ XDR-10 vs ReadyToProcess WAVE™ 25 is that scalability can be achieved with the Xcellerex™ XDR family up to 2000 L.
Scalable process for rAAV2, rAAV8, and rAAV9 production
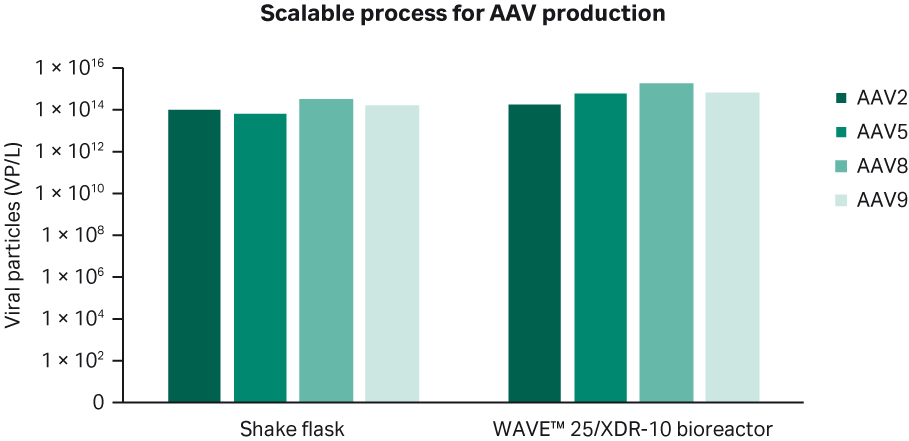
Research and development in the AAV field is highly active to improve efficacy and tissue specificity as well as to reduce some of the potential safety concerns. There are numerous natural as well as synthetic/engineered serotypes in various stages of development. We have also investigated whether our transfection protocol works for other serotypes than rAAV5 and is scalable.
Figure 7 shows good scalability and productivity from shake flask to bioreactor for rAAV2, rAAV8, and rAAV9 serotypes in addition to rAAV5. We conclude from these findings that the transfection protocol we describe for bioreactor scale up should allow production of different AAV serotypes at a relatively large scale.
Fig 7. Scalability of the transfection protocol for various AAV serotypes.
Conclusion and discussion
In this work, we present development of a closed bioreactor process for scalable production of AAV vectors that meet regulatory requirements for GMP production. The cell culture medium was serum-free, and no animal derived components were used. We completed three comparable rAAV5 production runs in XDR-10 bioreactor, fulfilling our acceptance criteria set for a complete upstream process. Our results show the robustness of the process and confirm that the harvest time at 72 h post-transfection is suitable for production. The results show good scalability from small scale in shake flasks up to 10 L scale in the XDR bioreactor.
In small-scale production, the advantages with performing the process in Xcellerex™ XDR-10 bioreactor compared to shake flasks is, for example, that the pH and DO is tightly controlled within the range of your choice.
In addition, we have shown AAV production in WAVE™ 25 up to 20 L with good results. The WAVE™ 25 bioreactor is relatively easy to operate and can provide material for AAV in early-stage preclinical development However, for large-scale GMP production, the Xcellerex™ XDR bioreactor family can be operated at up to 2000 L.
Importantly, the developed transfection protocol for rAAV5 also works for other AAV serotypes, such as AAV2, AAV8, and AAV9. We believe that the described production technology can be used for all natural or engineered serotypes with minor modification.
Cell culture of HEK293T suspension cells
We adapted adherent HEK293T cells to suspension culture in our HyClone™ transfection medium, HyCell™ Transfx-H. The HEK293T suspension cells were routinely passaged/subcultured three times per week at an inoculum VCD of 0.2 to 0.3 × 106 cells/mL. Cells were counted using Vi-CELL™ cell counter (Beckman-Coulter) and seeded in a new shake flask with fresh medium (E125/E250 flasks). Medium was supplemented with 4 mM L-glutamine and 0.1% Pluronic™ F-68 (BASF SE).
Transfection
rAAV5 was produced through transient transfection of HEK293T cells using a triple-plasmid system and with PEI max (Polysciences), enabling production of functional rAAV5 viral particles only in cells transfected with all three plasmids.
Briefly, the DNA solution was mixed in medium, and PEI reagent was added to the DNA mixture while gently swirling the transfer bottle. The PEI/DNA mix was incubated at room temperature for 15 min to form DNA complex. During incubation, the transfer bottle with the PEI/DNA mix was connected by welding the tubing to the bioreactor. An overpressure was created in the transfer bottle to transfer the transfection mix slowly into the bioreactor. Since we know that the incubation time is an important parameter to achieve a large share of full capsids in the harvest material, it is important to transfer the transfection solution in an efficient way to the bioreactor within 15 min.
After 72 h, samples from the bioreactor were collected and analyzed using flow cytometry to determine transfection efficiency. After lysis of the cells, a sample was taken and centrifuged at 850 × g for 10 min. An aliquot was prepared to be analyzed by qPCR for viral genome titer, and by ELISA for viral particles.
The final transfection protocol and parameters are described in Table 1 below:
Table 1. Final parameters used for the transfection protocol
| Parameters | rAAV |
| VCD (× 106/mL) | 1 |
| DNA concentration, total DNA (µg/mL)1 | 0.75 |
| DNA ratio: a:b:c | 1:1:2 |
| a | pAAV-RC5 Vector: 1 |
| b | pHelper: 1 |
| c | pAAV-GFP vector: 2 |
| PEI/DNA ratio (µL: µg) | 2:1 |
| Transfection volume | 5% of the total volume |
| Incubation time (min) | 15 |
| Temperature transfection | RT |
| Temperature AAV production | 37°C |
| Time of harvest, ToH (h) | 72 |
Using the optimized parameters in Table 1, the transfection protocol for production of rAAV5 in the bioreactors was as follows:
Day 0:
The bioreactor is inoculated with HEK293Tcell suspension at 0.4 × 106 cells/mL in 55% of the final culture volume.
Day 2, transfection:
- Add complete HyCell™ TransFx-H medium to final volume (10 L) at a cell density of 1 × 106 cells/mL.
- Prepare 0.75 μg/mL of total DNA in serum- and supplement-free medium to 5% of final production volume in a sterile flask.
- Add PEI (for a 1:2 DNA:PEI ratio) to the DNA mixture and mix by swirling the transfer bottle back and forth gently.
- Incubate for 15 min at room temperature.
- Connect the transfer bottle to the bioreactor by welding the tubing to the bioreactor and continue to create an overpressure in the transfer bottle to transfer the transfection mix slowly into the bioreactor.
Day 5, 72 h post-transfection:
- Collect samples and check transfection efficiency with flow cytometry and microscopy at 72 h post transfection, 37°C.
- Lyse the cells in the bioreactor by adding 1/10 of culture volume of lysis buffer (1.65 M NaCl, 5.5% Tween™ 20, 11 mM MgCl2) and incubate for 20 min.
- Continue adding 40 U DNAse/mL (Denarase™, c-LEcta GmbH) harvest and incubate for at least 4 h.
- Collect sample after lysing as a start reference sample. The remaining material is collected for further downstream processing.
XDR-10 bioreactor conditions
Parameter settings for the Xcellerex™ XDR-10 bioreactor for production of 10 L of rAAV5 are described in Table 2. The pH setpoint was set to 7.1 and controlled by CO2 and NaHCO3 using system default proportional–integral–derivative (PID) settings. DO set point was 40% and controlled by oxygen-enriched air, using the system default PID control settings.
Table 2. Summary of the parameter settings used for AAV5 production in XDR-10 bioreactor
| Parameters | Settings/comment XDR-10 bioreactor |
| Production medium | HyCell™ TransFx-H transfection medium (4 mM glutamine addition, 0.1% Pluronic™ F68) |
| Starting viable cell concentration | 0.4 × 106 viable cells/mL |
| Filling volume | 4.5 L |
| Inoculum volume | 1 L |
| Transfection mixture | 500 mL |
| Operating volume | 10 L |
| Impeller/rocker speed | 140 rpm, up pumping |
| Temperature | 37°C |
| pH set-point | 7.1 (controlled by CO2 and base) |
| Base for pH control | 7.5% (w/v) NaHCO3 |
| DO set point | 40 % (controlled by oxygen enriched air) |
| Spargers | 1 mm: air, CO2
2 µm: air, O2 |
| Oxygen flow | Proportional-integral-derivative (PID) controlled |
| Carbon dioxide flow | PID controlled |
| Antifoam | N/A |
ReadyToProcess WAVE™ 25 bioreactor
ReadyToProcess WAVE™ 25 bioreactor was prepared and run according to the user manual and checklist for ReadyToProcess WAVE™ 25. The bioreactor settings are listed in Table 7. The bioreactor used in this experiment contained the following components:
- 1 × ReadyToProcess WAVE™ 25 rocker
- 1 × ReadyToProcess™ CBCU
- 1 × 50 L Cellbag™ bioreactor (here called Cellbag™)
- UNICORN™ software
The procedure to produce 20 L of rAAV5 is described. Before inoculation, the 50 L Cellbag™ was inflated with air and 5 L of HyCell™ TransFx-H cultivation media was added aseptically to the bioreactor via one of the ports on the bag. Equilibration was performed overnight at 37°C and 5% CO2. The agitation settings were 15 rpm during equilibration. Before cell inoculation, an offset calibration of pH was performed.
The target for starting VCD was 0.4 × 106 cells/mL and the rpm increased to 20 rpm and angle 6°. After approximately 30 min, a sample was taken, and cell density was measured (start density) after which samples were taken every 24 h to determine cell density.
After two days, cells were diluted with pre-warmed HyCell™ TransFx-H medium supplemented with 4 mM L-glutamine and 0.1% Pluronic™ F-68 to reach a cell density of 0.8 to 1.2 × 106 cells/mL and a total volume of 20 L. Rocking was increased to approximately 22 rpm. This was followed by rAAV5 transfection and three days later, cells were harvested and subjected to lysis and clarification.
Table 3. Parameter settings used for production of 20 L of rAAV5 in WAVE™ 25 bioreactor
| Parameters | Settings, WAVE™ 25 bioreactor |
| Medium | HyCell™ TransFxH media (addition of 4 mM glutamine) |
| Starting viable cell concentration (VCD/mL) | 0.4 × 106 |
| Starting volume (L) | 5 to 6 |
| Inoculation volume (L) | 10 to 12.5 |
| Operating volume (L) | 20 to 25 |
| Transfection volume (% of total vol.) | 5% of total volume (1.00 to 1.25 L) |
| Rocking motion (rpm) | 15 to 25 rpm |
| Angle (°) | |
| Temperature | 37°C |
| Gas flow | 0.2 Lpm |
| pH set-point | 7.1 (controlled by CO2) |
| Base for pH control | N/A |
| DO set point | N/A (Not needed due to short culture time) |
| O2 | O2 concentration in gas mix was measured between 0% and 50% and controlled between 21% and 50% with air |
| CO2 | CO2 concentration in gas mix was controlled between 0% and 15% and measured between 0% and 20% |
Cell density and viability
Cell number and viability were measured using program HEK293.sus for Vi-CELL™ XR (Beckman Coulter). The principle of the method used by the instrument is trypan blue staining of cells and counting of dead (stained cells) and live cells (non-stained cells). NucleoCounter™ NC200™ Automated Cell Counter (Chemometec) was used for estimation of aggregates.
pH (offline)
Offline pH measurements were performed with SevenCompact S210 (Mettler Toledo™ Inc.). The pH meter was calibrated every day with pH 4.0 and pH 7.0 calibration solutions. The offline value was compared to the online value which was corrected if the difference was ± 0.1.
Morphology
Cell morphology and virus infection was visually monitored using Nikon Eclipse TE2000e inverted microscope (Nikon™ Corp.) with bright field for cell morphology. A fluorescence filter for detection of cells expressing the green fluorescent protein (GFP) was used to detect production of GFP-expressing virus.
Flow cytometry
Flow cytometry was used to rapidly analyze large numbers of individual cells or particles in suspension for different physical, chemical, or fluorescent characteristics. Here, the flow cytometry was used to evaluate the transfection efficiency, since transfected cells express GFP-protein. This is however only an indication of the fraction of cells transfected with the GFP-plasmid, and results do not necessarily correlate with viral titer in produced material.
qPCR
To determine the viral titer in samples a qPCR assay was used, by measuring viral genomes/mL in rAAV5 production. Primers for titer determination were targeting the CMV promoter region of the viral genome.
ELISA
rAAV5 titration ELISA kits from Progen were used for analysis of rAAV5 production.
CY24225-24Dec21-AN
TR29777055